Article Text
Abstract
Background Animal models have suggested that CCR2-dependent signalling contributes to the pathogenesis of pulmonary fibrosis, but global blockade of CCL2 failed to improve the clinical course of patients with lung fibrosis. However, as levels of CCR2+CD4+ T cells in paediatric lung fibrosis had previously been found to be increased, correlating with clinical symptoms, we hypothesised that distinct CCR2+ cell populations might either increase or decrease disease pathogenesis depending on their subtype.
Objective To investigate the role of CCR2+CD4+ T cells in experimental lung fibrosis and in patients with idiopathic pulmonary fibrosis and other fibrosis.
Methods Pulmonary CCR2+CD4+ T cells were analysed using flow cytometry and mRNA profiling, followed by in silico pathway analysis, in vitro assays and adoptive transfer experiments.
Results Frequencies of CCR2+CD4+ T cells were increased in experimental fibrosis—specifically the CD62L-CD44+ effector memory T cell phenotype, displaying a distinct chemokine receptor profile. mRNA profiling of isolated CCR2+CD4+ T cells from fibrotic lungs suggested immune regulatory functions, a finding that was confirmed in vitro using suppressor assays. Importantly, adoptive transfer of CCR2+CD4+ T cells attenuated fibrosis development. The results were partly corroborated in patients with lung fibrosis, by showing higher percentages of Foxp3+ CD25+ cells within bronchoalveolar lavage fluid CCR2+CD4+ T cells as compared with CCR2-CD4+ T cells.
Conclusion Pulmonary CCR2+CD4+ T cells are immunosuppressive, and could attenuate lung inflammation and fibrosis. Therapeutic strategies completely abrogating CCR2-dependent signalling will therefore also eliminate cell populations with protective roles in fibrotic lung disease. This emphasises the need for a detailed understanding of the functions of immune cell subsets in fibrotic lung disease.
- pulmonary fibrosis
- IPF
- CCR2+CD4 T Cell
- immunosuppressive
Statistics from Altmetric.com
Key messages
What is the key question?
What is the contribution of pulmonary CCR2+CD4+ T cells to the development of fibrotic lung disease?
What is the bottom line?
Murine CCR2+CD4+ T cells from fibrotic lungs are immunosuppressive and attenuate inflammation and fibrosis development when adoptively transferred: akin to the mouse, bronchoalveolar lavage fluid CCR2+CD4+Foxp3+ T cells are increased in patients with idiopathic pulmonary fibrosis.
Why read on?
We describe a novel pulmonary CCR2+CD4+ T cell subset that plays a protective role in lung fibrosis development: this has important implications for the development of treatment strategies targeting CCR2-dependent signalling.
Introduction
Pulmonary fibrosis is a manifestation of later stages of several interstitial lung diseases (ILDs), and is characterised by excessive deposition of extracellular matrix (ECM) within the lung interstitium. One of the the most common forms of ILD is idiopathic pulmonary fibrosis (IPF), with a poor mean survival time after diagnosis. Despite the recent development of novel pharmacological compounds that decelerate lung function decline,1 2 there is no cure in sight.
IPF is thought to result from repetitive epithelial injury followed by an inflammatory response and abnormal wound healing.3 This process is believed to result from a complex interplay between structural and various inflammatory cells, including lymphocytes. As anti-inflammatory therapies did not provide clinical benefit, immune cells were considered not to be important for disease pathogenesis. However, one clinical trial indicated that immunosuppressive therapy could be harmful for the patients,4 raising the question as to whether some specific immune functions might be protective in IPF.
Chemokine (C-C motif) ligand 2 (CCL2), is a major chemoattractant for several cell types, including macrophages and fibrocytes, but most strongly attracts T cells.5 Experimentally induced deficiency of its main receptor (CCR2) attenuated toxin-induced lung fibrosis in animal models.6–9 In patients with IPF, increased levels of CCL2 were found in serum,10 bronchoalveolar lavage fluid (BALF)9 and alveolar epithelium.11 These observations led to the recent clinical investigation of carlumab, an antibody blocking the CCR2 ligand CCL2, as treatment for human lung fibrosis.12 Surprisingly, complete blockade of CCL2 failed to demonstrate clinical advantage for these patients as measured by pulmonary function tests, time to disease progression and quality of life.
In an earlier study, we observed an increase of CCR2+CD4+ T cells in paediatric patients with ILD,13 the levels of which correlated positively both with disease severity and lung function. In addition, hyperoxia-induced lung injury was observed to be more severe in CCR2 knockout mice, indicating a multifaceted role of CCR2+ cells in lung injury.14 This prompted us to ask whether complete abrogation of all CCR2+ cell populations might also eliminate cells that protect from the disease. In particular, might increased pulmonary CCR2+CD4+ T cells either play no role in the pathogenesis and progression of ILD, or even represent an attempt of the immune system to counteract the disease?
If the latter holds true, this would require the development of more targeted therapeutic strategies. Therefore, the aim of this study was to investigate the specific role of pulmonary CCR2+CD4+ T cells in lung fibrosis.
Methods
Please refer to the ‘Methods’ section in this article’s online repository for a detailed description of the study designs for animals and patients, statistical analyses, mRNA arrays, suppression assays, cell preparation for the transfer experiments and lung function analysis.
Animal treatment
All animal studies were conducted under the Federal guidelines for the use and care of laboratory animals and were approved by the government of the District of Upper Bavaria.
Specific pathogen-free female C57BL/6 mice, 7–8 weeks old (Charles River Laboratories, Sulzfeld, Germany), were used for the study. We used the bleomycin model, the most commonly employed model for IPF in rodents. For all analyses, a single dose of bleomycin (5 U/kg body weight in 200 µL sterile phosphate-buffered saline (PBS), Sigma-Aldrich, Seelze, Germany) was administered orotracheally under anaesthesia on day 0 using MicroSprayer MS-IA-1C (Penn-Century, Wyndmoor, USA), as previously reported.15 For adoptive transfer experiments, 1.5 U/kg body weight was used, with purified CCR2+ or CCR2-CD4+ T cells suspended in 50 µl sterile PBS and instilled intratracheally, as previously described.16
Murine cell preparation and flow cytometry (FACS) analysis
Animals were sacrificed at 3-day intervals up to day 21 after bleomycin instillation. Single-cell suspensions of lung, spleen and BAL cells were obtained at each time point. Briefly, 1 mL of PBS was instilled into lungs and lavaged three times. The BALF and cells were collected after centrifugation (400 g, 10 min at 4°C). After being carefully dispersed into small pieces, lung tissue was incubated with digestion buffer containing RPMI 1640 (Invitrogen, Darmstadt, Germany), 0.07% collagenase A (Roche, Mannheim, Germany) and 0.01% DNase (AppliChem, Darmstadt, Germany) at 37°C for 40 min.
Samples were stained with CD3 pacific blue (Biolegend, San Diego, USA), CD4 APC-H7 (BD-Biosciences, Heidelberg, Germany), CCR2 APC (R&D), CD62L PE (Biolegend), CD44 FITC (Biolegend), and different CCRs antibodies (CCR3 and 10, CXCR4, 5 and 6 antibodies are from R&D, CCR4, 6, 7 and 9, CXCR3 antibodies are from Biolegend). Intracelluar staining for Foxp3 (eBioscience, San Diego, USA), interleukin (IL)-10 (Biolegend) and IL-13 (eBioscience) was performed according to the manufacturers’ protocols. For cytokine staining, lung cells were first stimulated with phorbol 12-myristate 13-acetate (5 ng/mL), ionomycin (500 ng/mL) and brefeldin A (10 µg/mL) (Sigma-Aldrich, Seelze, Germany) at 37°C for 4 hour.
Cells were analysed with a flow cytometer (LSRII, BD, Heidelberg, Germany) and BD FACSDiva software. The percentages of CCR2 and other chemokine receptors, IL-10 and IL-13 were determined and compared with their respective isotype controls.
Pathway, function and network analysis
The top canonical pathways, function and network analyses were generated through the use of IPA (ingenuity pathway analysis (Qiagen)). First, the core analysis module of IPA was applied to a set of 977 mRNAs that were differentially regulated in CCR2+compared with CCR2-CD4+ T cells (|FC|>1.5, p<0.05) using the following settings: ingenuity knowledge base (genes only) relationship to include: direct and indirect, includes endogenous chemicals; filter summary: consider only relationships where confidence = experimentally observed. The mouse gene 2.1 ST array was used as reference set. Then, as sorted lymphocyte subsets were analysed, we focused on ‘lymphoid tissue structure and development’ for further function and network analysis. The functional analysis of a network identifies the biological functions that are most significant to the genes in the network. A Fisher’s exact test was used to calculate a p value determining the probability that each biological function in that network is due to chance alone.
Histology
Lungs were fixed in 4% formaldehyde and embedded in paraffin. Sections (3 µm) were obtained from blocks and mounted on coated glass slides, deparaffinised with xylene and graded ethanol. The sections were stained with Goldner’s Masson trichrome (Sigma-Aldrich, Seelze, Germany) by a standard technique.
Patients
BAL samples were obtained from treatment-naïve patients who underwent bronchoscopy for diagnostic purposes. The study was approved by the institutional review board of the University of Munich and written informed consent from patients was obtained before examination.
Diagnosis of IPF was made according to the current American Thoracic Society/European Respiratory Society consensus statement.17 We included consecutive patients from the Department of Respiratory Medicine, Asklepios Clinic Gauting, Germany with IPF and other pulmonary fibrosis, who underwent BAL on clinical indication outside of infection or exacerbation. Patients with lung fibrosis other than IPF (non-IPF) included those with non-specific interstitial pneumonitis, combined pulmonary fibrosis and emphysema, hypersensitivity pneumonitis, connective tissue disease, amiodarone-induced lung fibrosis and fibrosis that could not be further classified. Consecutive patients with non-fibrotic lung diseases were included as disease controls (chronic bronchitis, COPD, neoplasia).
Immunophenotyping
Human BALF cells were stained with the following antibodies after erythrocyte lysis (Lysis buffer, BD Biosciences, Heidelberg, Germany): CD3-PerCP, CD25 FITC, CCR2 APC (all BD Biosciences) CD4–PB (Biolegend, San Diego, USA). Intracellular forkhead box p3 (Foxp3) was stained (anti-Foxp3-PE, eBioscience, San Diego, USA) using an intracellular staining kit, according to the manufacturer’s instructions (BD Pharmigen, San Diego, USA). Cells were analysed with a flow cytometer (LSRII, BD-Biosciences, Heidelberg, Germany) and Flowjo software (vX, Tristar, Ashland, USA). Percentages of CCR2 were determined and compared with their respective isotype controls. Of note, CD25 was stained as a marker of Tregs in the human samples, whereas in the mouse studies, functional assays were used to confirm the regulatory phenotype.
Statistical analysis
All results are expressed as mean and SEM. Normal distribution was tested by Kolgorov–Smirnov test; parametric tests were used accordingly as specified in the respective figure legends. Significance level α=0.05 was used throughout the study and * symbolises values of p<0.05 in the figures (GraphPad Prism 7, La Jolla, USA). For further details on design and statistical analyses of each experiment please refer to the corresponding figure legends and the online repository included in online supplementary tables S2–S7.
Results
CCR2+CD4+ T cell levels in experimental lung fibrosis
To investigate the kinetics of CCR2+CD4+ T cells after bleomycin treatment and correlate their course with fibrosis development, lung and BALF cells were analysed by fluorescence-activated cell sorting (FACS) at various time points after bleomycin instillation, starting at day 3 until day 21. Enhanced collagen deposition was observed in the lungs beginning at day 6 and becoming more pronounced from day 12 onwards until day 21 (figure 1A). At day 12 mean±SEM total BALF cell numbers were 66.80±14.48×104 (figure 1B), and the percentage of pulmonary CCR2+CD4+ T cells in BALF was 20.8±2.7% (figure 1C). The mean±SEM percentages of pulmonary CCR2+CD4+ T cells were increased from day 6 (9.1±1.3% compared with 5.3±1.0% in control mice) reaching a maximum at day 12 (16.3±2.7%) (figure 1D and E). Since this is the most relevant endpoint, day 12 was chosen as time point for readout in further experiments.
Transcriptomic profiling of CCR2+CD4+ T cells from fibrotic lungs shows increased expression of markers of differentiation of regulatory T cells. Pulmonary CCR2+ and CCR2-CD4+ T cells were sorted at day 12 after bleomycin instillation. mRNA arrays of individual samples were performed. The heatmap represents the expression values of 1680 genes (false discovery rate <10%), which were regulated in CCR2+CD4+ T cells compared with CCR2-CD4+ T cells (A). Ingenuity pathway analysis of the mRNA array data showed ‘T helper cell differentiation’ as the top canonical pathway (B). In the functional analysis the network ‘differentiation of regulatory T lymphocytes’ (C) showed the highest significant activation score (activation z-score 2.469; p value 2.13×10−7). p Values were calculated by Fisher’s exact test determining the probability that the biological function of that network is due to chance alone. Gene expressions of Foxp3 and interleukin 10 (IL-10) (D) were significantly increased, by 3.59- and 5.28-fold, respectively, in CCR2+CD4+ T cells compared with CCR2-CD4+ T cells. Data represent the mean ± SEM from five animals. *p<0.05 versus CCR2-CD4+ T cells by t test.
Phenotypes of CCR2+CD4+ T cells in fibrotic lungs
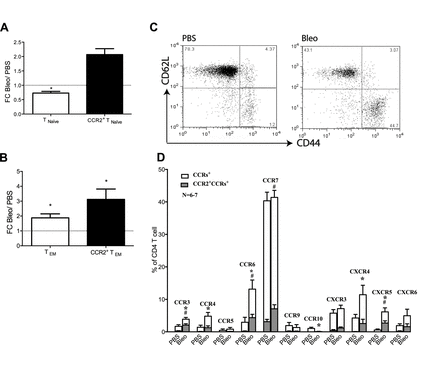
To investigate whether the newly recruited T cells are naïve or antigen-experienced, CD62L and CD44 were used as markers to differentiate between effector memory and naïve T cells. The frequency of CD62L+CD44- naïve T cells (TNaïve) decreased in bleomycin-treated animals compared with PBS controls from 70.8±3.8% to 51.6±4.2% (ratios shown in figure 2A,) while the frequency of CD62L-CD44+ effector memory T cells (TEM) nearly doubled (17.2%±1.8% to 32.3±4.6%, ratios in figure 2B). Notably, the percentage of CCR2+ TEM was significantly increased within CD4+ T cells (12.5%±2.7% compared with 3.9%±0.9% in control mice), and the relative increase in TEM was more pronounced within the CCR2+ population (ratios shown in figure 2B, 3.1-fold increase, representative FACS dot plots shown in figure 2C) than in the total CD4+ T cells (1.8-fold increase), indicating that the majority of CCR2+CD4+ T cells were TEM.
Kinetics of pulmonary CCR2+CD4+ T cells at day 12 after bleomycin treatment. Naïve T cells (CD62L+CD44-) and effector memory T cells (CD62L-CD44+) were gated within CD4+ T cells. The population of naïve T cells (TNaïve) decreased (A) while that of effector memory T cells (TEM) increased (B) at day 12 after bleomycin treatment. CCR2+ TEM were significantly upregulated within CD4+ T cells with a fold change (FC) of 3.1 (B). Representative fluorescence-activated cell sorting dot plots gated on CD4+ cells are shown in (C). Expressions of CCR3, −6,–7 and CXCR5 were increased on CCR2+CD4+ T cells after bleomycin treatment compared with phosphate-buffered saline (PBS)-treated mice (D). Data represent the mean ± SEM from six to seven animals. (A+B): Fold changes calculated from percentages of TNaive or TEM within CD4+ T cells at day 12 after bleomycin versus PBS. (D) *p<0.05 versus PBS (percentage CCRs+ gated within CD4+ T cells) and # p<0.05 versus PBS (percentage of CCRs+ gated within CCR2+CD4+ T cells) by t test.
In contrast, CD62L+CD44+ central memory T cells were a minor population (<5%) within CD4+ T cells (figure 2B) and <1% naïve T cells were also CCR2+ both in control and bleomycin-treated mice (data not shown).
To obtain information on the migratory potential of CCR2+ T cells, we further investigated the co-expression of other chemokine receptors on CCR2+CD4+ T cells. In fibrotic lungs, the frequencies of CCR3, −6,–7 and CXCR5-positive cells within CCR2+CD4+ T cells were significantly increased as compared with healthy lungs. The frequencies of CCR4, CXCR4 and CXCR6-positive cells within the total CD4+ T cells were significantly increased in fibrotic compared with healthy lungs, but this increase was not significant within the CCR2+CD4+ T cells (figure 2D). CCR5+, CCR9+ and CCR10+ CCR2+CD4+ T cells were present at low levels, with a decrease of CCR10+ CCR2+CD4+ T cells in bleomycin-treated mice.
Upregulation of Foxp3 and IL-10 in CCR2+CD4+ T cells from fibrotic lungs
As these results indicated the existence of a distinct phenotype of CCR2+CD4+ T cells, we performed transcriptomic profiling of CCR2+CD4+ and CCR2-CD4+ T cells from fibrotic lungs to gain insight into their potential functional characteristics.
In total, of 28 442 probe sets on the chip, 1680 probes were differentially regulated in CR2+CD4+ T cells compared with CCR2-CD4+ T cells (false discovery rate <10%). Of these, 977 genes were changed at least 1.5-fold (figure 3A and online Supplementary Table 1). The expression of CCR2 was 9.7-fold higher in CCR2+CD4+ T cells, thus confirming the high purity of sorted CCR2+CD4+ T cells. The highest fold changes were observed for killer cell lectin-like receptor subfamily G member 1 (klrg1; 6.6-fold), histocompatibility 2, class II antigen A alpha (6.3-fold), CCR3 (5.9-fold), and fibrinogen-like protein 2 (fgl2, 5.5-fold) and interleukin 10 (IL-10, 5.3-fold, online Supplementary Table 1). The IPA core analysis (IPA, Qiagen Redwood City, USA) of significantly regulated genes revealed T helper cell differentiation, ICOS (inducible co-stimulator)–ICOSL signalling in T helper cells, role of nuclear factor of activated T cells in regulation of the immune response and IL-10 signalling as the top canonical pathways (figure 3B). In an IPA function analysis of ’ lymphoid tissue structure and development’, the highest activation score was found for ‘differentiation of regulatory T lymphocytes (Treg)’ (activation score 2.469, p value=2.13×10-7). Within this function network (figure 3C), the top regulated genes were Foxp3 (3.6-fold) and IL-10 (5.3-fold) (figure 3D). These findings were confirmed by intracellular staining of Foxp3 and IL-10. At day 12 after bleomycin treatment, Foxp3 and IL-10 expressing CD4+ T cells were significantly increased compared with control animals (6.8%±0.2% to 13.8±0.5% vs for Foxp3, 1.7±0.8% to 4.2%±0.7% for IL-10) (figure 4A-D). However, the frequencies of Foxp3 and IL-10 were significantly higher (3.8- and 2.8-fold, respectively) in CCR2+CD4+ T cells compared with CCR2-CD4+ T cells (figure 4E-H) in the bleomycin-induced lung fibrosis model.
Phenotypes of CCR2+CD4+ T cells at day 12 after bleomycin treatment. Naïve T cells (CD62L+CD44-) and effector memory T cells (CD62L-CD44+) were gated within CD4+ T cells. The population of naïve T cells (TNaïve) decreased (A) while that of effector memory T cells (TEM) increased (B) at day 12 after bleomycin treatment. CCR2+ TEM were significantly upregulated within CD4+ T cells with a fold change (FC) of 3.1 (B). Representative fluorescence-activated cell sorting dot plots gated on CD4+ cells are shown in (C). Expressions of CCR3, −6,–7 and CXCR5 were increased on CCR2+CD4+ T cells after bleomycin treatment compared with phosphate-buffered saline (PBS)-treated mice (D). Data represent the mean ± SEM from six to seven animals. (A+B) Fold changes calculated from percentages of TNaive or TEM within CD4+ T cells at day 12 after bleomycin versus PBS. (D) *p<0.05 versus PBS (percentage CCRs+ gated within CD4+ T cells) and #p<0.05 versus PBS (percentage of CCRs+ gated within CCR2+CD4+ T cells) by t test.
Intracellular staining of Foxp3 and interleukin 10 (IL-10) at day 12 after bleomycin treatment. In addition to extracellular staining with CD3, CD4 and CCR2, lung single cells were stained intracellularly with Foxp3 and IL-10 antibodies. Foxp3 (A+B) and IL-10 (C+D) expressing CD4+ T cells were increased in fibrotic lungs. The frequencies of Foxp3 (E+F) and IL-10 (G+H) were significantly higher in CCR2+ CD4+ T cells than in CCR2- CD4+ T cells in bleomycin mice, with a similar tendency for Foxp3 observed in control mice (although not significant for IL-10). Foxp3 and IL-10 were gated under CD4+ T cells (A–D) and CCR2+/CCR2-CD4+ T cells (E–H). Data represent the mean ± SEM from 6 to 7 animals in A and C, and 8 (PBS) or 13 (Bleo) animals in E and G. B, D, F and H are representative histograms underlying the respective bar diagrams A, C, E and G. #p<0.05 and versus control by t test and *p<0.05 versus CCR2-CD4+ T cells by analysis of variance with Bonferroni post-test.
Immunosuppressive function of pulmonary CCR2+CD4+ T cells
The high expression of Foxp3 and IL-10 in pulmonary CCR2+CD4+ T cells indicated a regulatory function of these cells in lung fibrosis. To test this hypothesis, we performed a suppression assay using stimulated CD4+ T cells as effector T cells (Teff). CD4+ T cells proliferated strongly under stimulation with CD3 (approximately 40% of the precursor cells), in the absence of natural Treg (nTreg) cells, or CCR2+ or CCR2-CD4+ T cells. nTreg (CD25highCD4+) cells inhibited the proliferation of Teff, with a maximum at the ratio of 1:1. A similar effect was observed in CCR2+CD4+ T cells, resulting in 30% inhibition at the ratio of 1:1 (figure 5A and B). In contrast, CCR2-CD4+ T cells did not affect Teff proliferation at any tested concentration. Owing to the small amount of pulmonary CCR2+CD4+ T cells in control animals, the suppression assays could not be performed with cells from ‘healthy’ mice.
Suppression assay of sorted CCR2+ and CCR2-CD4+ T cells from fibrotic lungs. Carboxyfluorescein succinimidyl ester-labelled CD25-CD4+ T cells (Teff cells) were cultured for 3 days in the absence or presence of CD25highCD4+ T (nTreg) cells, CCR2+ cells, or CCR2- CD4+ T cells, under stimulation with CD3 and CD25-CD4+ T cells. Cell proliferation was analysed by fluorescence-activated cell sorting (FACS). nTreg cells concentration dependently inhibited the proliferation of Teff cells (B). A similar effect was observed for CCR2+CD4+ T cells but not for CCR2-CD4+ T cells. Typical FACS pictures (A). Data represent the mean±SEM from two to three independent experiments. *p<0.05 versus CCR2-CD4+ T cells (1:1) by analysis of variance with Bonferroni post-test.
CCR2+CD4+ T cells and development of bleomycin-induced lung fibrosis
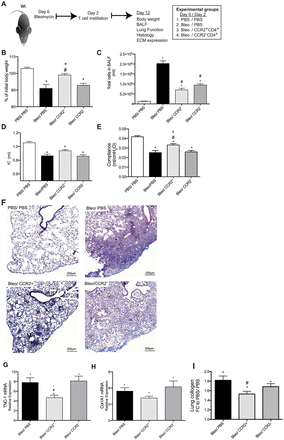
To investigate whether the immune regulatory properties of CCR2+CD4+ T cells are relevant in vivo, adoptive transfer of sorted cells was performed at day 2 after bleomycin treatment and analysed at day 12 (figure 6A). Animals treated with CCR2+CD4+ T cells exhibited less pronounced weight loss than animals that received bleomycin treatment solely (figure 6B). Further, inflammatory cells in BALF were reduced (figure 6C). Lung compliance was higher in animals receiving CCR2+CD4+ T cells than in animals treated with CCR2-CD4+ T cells or PBS (figure 6D), while the effect on inspiratory capacity (IC) was not significant (figure 6E). On histology, decreased cellular infiltrate and ECM deposition were observed in the animals treated with CCR2+CD4+ T cells (figure 6F). Relative expression of tenascin-C mRNA (figure 6G) in lung homogenate was significantly decreased in animals treated with CCR2+ CD4+ T cells and a similar trend was observed for collagen1a1 mRNA levels (figure 6H). Likewise, lung collagen content quantified by Sircol assay was decreased (figure 6I). None of these effects was seen after adoptive transfer of CCR2-CD4+ T cells. We next investigated the effect of CCR2+CD4+ T cells, when fibrosis is already installed. Fibrotic animals treated with PBS or CCR2-CD4+ T cells were significantly worse than non-fibrotic controls (PBS/ PBS) as assessed by BAL cell numbers, lung compliance and tenescin-1 expression (figure 7). In contrast, fibrotic animals treated with CCR2+CD4+ T cells were not significantly different from non-fibrotic controls.
Adoptive transfer of CCR2+ and CCR2-CD4+ T cells at day 2 for early intervention in bleomycin-induced lung fibrosis (A). Body weight loss during lung fibrosis development was less pronounced in animals treated with CCR2+CD4+ T cells (B). Total cells in bronchoalveolar lavage fluid (BALF) after bleomycin administration were decreased in animals treated with CCR2+ and CCR2-CD4+ T cells compared with phosphate-buffered saline (PBS)-treated animals (C). Inspiratory capacity (IC) (D) was not significantly changed by CCR2+CD4+ T cell treatment while compliance (E) was significantly ameliorated. On histology using Masson trichrome staining, decreased inflammation and ECM deposition were observed in the animals treated with CCR2+CD4+ T cells (F). Relative mRNA expression compared with controls (PBS/PBS) of tenescin-1 (TNC-1) (G) and collagen1a1 (Col1A1) (H) was increased in all bleomycin-treated animals. CCR2+CD4+ T cell treated animals showed a significant decrease of TNC-1 mRNA expression compared with Bleo/PBS and Bleo/CCR2- treated animals. (I) Soluble lung collagen content measured by Sircol assay. Relative collagen content was increased compared with controls (PBS/ PBS) in all animals. Animals treated with CCR2+CD4+ T cells showed a significantly lower lung collagen deposition than those treated with Bleo/PBS. Data represent mean±SEM from 6–12 animals/experimental group. *p<0.05 versus control (PBS/PBS), #p<0.05 versus Bleo/PBS, +p<0.05 vs Bleo/CCR2- by analysis of variance with Bonferroni post-test.
Adoptive transfer of CCR2+ and CCR2-CD4+ T cells at day 12 for treating bleomycin-induced lung fibrosis (A). Body weight loss had recovered at day 22 in all groups except for those treated with CCR2-CD4+ T cells (B). Total bronchoalveolar lavage fluid (BALF) cells did not differ significantly between animals treated with CCR2+CD4+ T cells and non-fibrotic control animals. In contrast, animals treated with phosphate-buffered saline (PBS) (sham) or CCR2-CD4+ T cells had significantly increased total BALF cells compared with non-fibrotic animals (C). Inspiratory capacity (IC) (D) and compliance (E) did not differ significantly between animals treated with CCR2+CD4+ T cells and non-fibrotic control animals but were significantly reduced in fibrotic animals treated with PBS or CCR2-CD4+ T cells. On histology using Masson trichrome staining, fibrotic changes appeared milder in the animals treated with CCR2+CD4+ T cells than in the other groups of fibrotic animals (F). Relative mRNA expression of enescin-1 (TNC-1) did not differ significantly between animals treated with CCR2+CD4+ T cells and non-fibrotic control animals (PBS/PBS) but was increased in fibrotic animals treated with PBS or CCR2-CD4+ T cells(G). A similar tendency was observed for collagen1a1 (Col1A1) (H). Data represent mean±SEM from 6–12 animals experimental group. *p<0.05 versus control (PBS/PBS) by analysis of variance with Bonferroni post-test.
Phenotypes of CCR2+CD4+ T cells in BALF from human patients with lung fibrosis
To examine to what extent the findings from the animal model are transferable to humans, we studied BALF from adult patients with IPF, non-IPF lung fibrosis and non-fibrotic lung diseases. Baseline characteristics of the patients are shown in table 1. As expected from our previous work in children,13 the percentages of CCR2+CD4+ T cells were increased in patients with IPF (4.71±0.97%) and other fibrotic lung diseases (4.13±0.78%) as compared with patients with non-fibrotic lung diseases (0.86±0.84%) (figure 8A and B). Further, higher percentages of Foxp3+CD25+CD4+ T cells were found in patients with IPF than in the control patients (figure 8C and D). In BALF from patients with IPF and non-IPF, the frequency of Foxp3+CD25+ cells was higher (3.8- and 2.5-fold, respectively) within CCR2+CD4+ T cells than within CCR2-CD4+ T cells (figure 8E and F). The small cell numbers prevented the performance of suppressor assays. In the control group there was a similar trend, but this analysis again was not possible for all samples owing to low numbers of CCR2+CD4+ T cells.
CCR2+CD4+ T cells in bronchoalveolar lavage fluid (BALF) of patients with idiopathic pulmonary fibrosis (IPF), non-IPF and controls. CCR2+CD4+ T cells (A) were increased in BALF of patients with IPF and other fibrosis (non-IPF) compared with patients with non-fibrotic diseases (controls). Foxp3+CD25+ T cells were increased in patients with IPF compared with controls (C). The frequencies of Foxp3+CD25+ T cells among CCR2+CD4+ T cells were increased as compared with CCR2-CD4+ T cells in patients with IPF and non-IPF (E). Foxp3+CD25+ cells were gated within CD4+ T cells (C+D) and within CCR2+/CCR2-CD4+ T cells (E+F). Data in A, C and E represent the mean ± SEM from n=10 (IPF), n=15 (non-IPF) and n=12 (control). Representative histograms and dot plots are shown in B + D (IPF and control) and F (IPF), underlying the bar graphs in A, C and E, respectively. #p<0.05 IPF or non-IPF versus control; +p<0.05 IPF vs non-IPF; *p<0.05 CCR2+ vs CCR2- within control, IPF or non-IPF; all by analysis of variance with Bonferroni post-test.
Patient characteristics
Discussion
Inflammatory cells, including CD4+ T cells,19 are typically found in fibrotic lung diseases, but their contribution to disease pathogenesis and progression in IPF has been questioned, mainly because anti-inflammatory treatment did not show a clinical benefit.4 In particular, in a recent study targeting of CCR2+ signalling with a non-specific CCL2 monoclonal antibody did not improve the clinical outcomes of IPF patients, with an unexpected increase in free CCL2 levels in patients receiving active treatment.12 The authors of that study hypothesised that this might in part be due to compensatory excess release of free CCL2.12 However, it is also possible that the lack of clinical benefit from the CCL2 monoclonal antibody might be because untargeted treatments not only eliminate disease promoting CCR2+ cell populations, but also those that potentially limit disease progression.
In this study, we confirmed increased frequencies of a CCR2+ subset of CD4+ T cells in BALF from patients with IPF, non-IPF lung fibrosis and in mice after induction of experimental fibrosis. Transcriptomic profiling of isolated murine CCR2+CD4+ T cells from fibrotic lungs indicated that this rare T cell subset exerts immune regulatory functions. This was corroborated in vitro where CCR2+CD4+ T cells suppressed T cell proliferation. A role of CCR2+CD4+ T cells in disease pathogenesis was further demonstrated by adoptive transfer experiments that resulted in attenuated lung inflammation and reduced fibrosis development.
Studies investigating discrete T cell subpopulations indicated that CCR7+ or CCR4+ T cells, or T cells responding to the ligands of these receptors (CCL18, CCL17), aggravate experimental lung fibrosis.20 In the present study, CCR2+CD4+ T cells displayed a distinct repertoire of the homing receptors CCR3, −6,–7 and CXCR5 after bleomycin treatment, suggesting the induction of a broad migratory potential. CCR3+ T cells have so far mainly been implicated in airway remodelling in asthma, but not in fibrotic disease.21 22 Furthermore, CCR3 and also CCR6 were found on subpopulations of effector/memory-like Treg and mediated their migration to inflammatory tissues.23–25 One study observed CCR7+ T cells at very rare frequencies in lungs from patients with IPF.26 Small numbers of Tregs expressing CXCR5 have been described in secondary lymphoid tissues. Thus, the chemokine receptor repertoire of CCR2+ CD4+ cells is similar to that of Treg, but its role in fibrotic disease has not been investigated so far. Given the low frequency of CCR2+CD4+ T cells, the expression of homing receptors on these cell subsets might have been overlooked in previous studies.
To obtain insight into the specific functions of CCR2+CD4+ T cells we performed mRNA arrays in CCR2+ and CCR2-CD4+ T cells isolated from fibrotic murine lungs. IL-10, together with Klrg1 and fgl2 were among the top regulated genes of the array. Similar to IL-10, fgl2 is a secreted Treg effector molecule pivotal for maintaining peripheral immune tolerance.27 28 Surface expression of Klrg1+ is a marker of highly active but short-lived terminally differentiated Treg cells,29 which suggests that CCR2+CD4+ T cells can be effective even when present in small numbers. Of note, Klrg1+ Treg cells act predominantly to maintain immune homoeostasis in mesenchymal tissues.30 So far, the role of conventional Foxp3+ Treg cells in lung fibrosis has remained unclear: while some animal31–33 and human34 35 studies suggest that Treg contribute to fibrosis development—for example, via transforming growth factor β or platelet-derived growth factor production, others found evidence for a limitation of fibrosis by Treg.20 36–38 Similarly, the increased IL-10 expression in lung fibrosis demonstrated in many studies39 40 may have anti-inflammatory and thereby fibrosis-limiting effects, but may also promote fibrosis.41 42 The conflicting results could be explained by the use of different disease models or stages,16 43 and—more importantly—could be linked to specific subsets such as CCR2+CD4+ T cells.
Using IPA core analysis, we identified four top canonical pathways that were exclusively related to T cell differentiation and activation. Among these, increased inducible T cell co-stimulator (ICOS) and IL-10 signalling were found in pulmonary CCR2+CD4+ T cells. Decreased expression of costimulatory signals in peripheral blood mononuclear cells, including ICOS, was associated with shorter transplant-free survival of patients with IPF,44 suggesting that these molecules confer protective functions in fibrosis progression. Furthermore, IPA function analysis revealed ‘differentiation of regulatory T cells’ as the function with the highest activation score. Here IL-10 and the transcription factor Foxp3 were most strongly upregulated, driving differentiation and function of Treg cells. Besides Foxp3, a transcription factor termed nuclear receptor subfamily four group A member 1 (NR4A1), which plays an essential role in regulatory T cell development,45 was also increased. Further, NR4A1 is an important inhibitor of transforming growth factor β signalling in the mesenchyme and prevents fibrosis development.46 Indeed, in our study CCR2+CD4+ T cells from fibrotic lungs showed suppressor activity in vitro. This is important as in an earlier study conventional Treg cells were found to be reduced and silenced in lungs of patients with IPF.35 In contrast, blockade of CCR2 during progression of collagen-induced arthritis aggravated clinical and histological disease scores, owing to the inhibition of CCR2+CD4+ T cells displaying regulatory function during the disease course.47
Nonetheless a number of questions arise: adoptive transfer of cells after bleomycin application limited lung injury, but this was performed at an early disease stage (in this murine model) where inflammation predominates. Treatment at a later time point, when fibrosis is already installed, was less effective but still ameliorated fibrosis and lung function impairment. Limitations of the bleomycin model include the marked initial inflammation and also the spontaneous resolution: this makes it difficult to define a disease phase that perfectly matches human disease in its symptomatic (ie, later) phase. Moreover, fibrotic disease in mice is a continuum of events, starting with inflammation followed by collagen deposition and finally, resolution of fibrosis, whereas in human IPF, inflammation and fibrosis develop in parallel. Thus, it is conceivable that CCR2+CD4+ T cells might also act at later stages of the disease in humans. Yet, functional assays were not possible using human cells owing to the limited number of cells available, and therefore CD25 was used as an additional marker of regulatory phenotype in the human study.
In summary, this study provides evidence for anti-inflammatory and anti-fibrotic properties of CCR2+CD4+ T cells, and more importantly demonstrates that the contribution of discrete T cell subsets to lung fibrosis is more complex than previously thought. This emphasises the need for detailed studies to enable the development of new targeted therapeutic strategies, and helps to explain previous clinical data.
Acknowledgments
We thank Dr Faisal Maqbool Zahid for statistical support; Dr Ina Koch and the Asklepios Biobank Gauting, Member of the German Centre for Lung Research, for the preprocessing and provision of the patient samples and Ms Qihui Zhou for technical support with the suppression assay.
References
Footnotes
Contributors Acquisition of data: KM, YY, EB, MI, AS, MM and ML; conception, design, supervision: SK-E; analysis and interpretation: SK-E, KM, YY, EB, JB, MI, OE, MK; recruitment of patients and human sample collection: FR and JüB; wrote manuscript: SKE, KM and YY; approved and edited manuscript: OE, MK, JüB, FR, MI, JB, ML, AS, EB and MM.
Funding This work was supported by the German ResearchFoundation (1973 4-1).
Competing interests KM, MK, OE and SK-E are partners of the European COST (Cooperation in Science and Technology) Action BM1201 ‘Developmental Origins of Chronic Lung Disease’. Other authors have no competing interests to declare.
Ethics approval Institutional review board, University of Munich.
Provenance and peer review Not commissioned; externally peer reviewed.