Article Text
Abstract
Background Millions of individuals with obstructive sleep apnoea (OSA) are treated by CPAP aimed at reducing blood pressure (BP) and thus cardiovascular risk. However, evidence is scarce concerning the impact of different CPAP modalities on BP evolution.
Methods This double-blind, randomised clinical trial of parallel groups of patients with OSA indicated for CPAP treatment compared the efficacy of fixed-pressure CPAP (FP-CPAP) with auto-adjusting CPAP (AutoCPAP) in reducing BP. The primary endpoint was the change in office systolic BP after 4 months. Secondary endpoints included 24 h BP measurements.
Results Patients (322) were randomised to FP-CPAP (n=161) or AutoCPAP (n=161). The mean apnoea+hypopnoea index (AHI) was 43/h (SD, 21); mean age was 57 (SD, 11), with 70% of males; mean body mass index was 31.3 kg/m2 (SD, 6.6) and median device use was 5.1 h/night. In the intention-to-treat analysis, office systolic blood pressure decreased by 2.2 mm Hg (95% CI −5.8 to 1.4) and 0.4 mm Hg (−4.3 to 3.4) in the FP-CPAP and AutoCPAP group, respectively (group difference: −1.3 mm Hg (95% CI −4.1 to 1.5); p=0.37, adjusted for baseline BP values). 24 h diastolic BP (DBP) decreased by 1.7 mm Hg (95% CI −3.9 to 0.5) and 0.5 mm Hg (95% CI −2.3 to 1.3) in the FP-CPAP and AutoCPAP group, respectively (group difference: −1.4 mm Hg (95% CI −2.7 to −0.01); p=0.048, adjusted for baseline BP values).
Conclusions The result was negative regarding the primary outcome of office BP, while FP-CPAP was more effective in reducing 24 h DBP (a secondary outcome).
Trial registration number NCT01090297.
- Sleep apnoea
Statistics from Altmetric.com
Key messages
What is the key question?
Is fixed pressure CPAP superior to auto-adjusting CPAP in reducing blood pressure in patients with obstructive sleep apnoea (OSA)?
What is the bottom line?
In this double-blind, randomised clinical trial of parallel groups involving 322 patients with OSA indicated for CPAP treatment, although fixed pressure and auto-adjusting CPAP had similar impact on clinical blood pressure (primary outcome), fixed pressure CPAP was more effective than auto-adjusting CPAP in reducing 24 h diastolic blood pressure (secondary outcome).
Why read on?
The interest of fixed pressure CPAP for reducing 24 h blood pressure might be of particular clinical relevance for patients with OSA, with poorly controlled or resistant hypertension.
Introduction
Obstructive sleep apnoea (OSA) is characterised by recurrent pharyngeal collapses occurring during sleep. OSA is a chronic disease, as frequent as asthma, affecting 5%–15% of the general population, and steadily worsens up to age 60.1 ,2 OSA has a dose–response relationship with hypertension.3 ,4 Hypertension is among the factors that contribute to cardiovascular morbidity and mortality in OSA,5 ,6 increasing the risk of fatal and non-fatal cardiovascular events,5 such that OSA represents an independent risk factor for sudden cardiovascular death.7
CPAP remains the first-line therapy for OSA. Randomised controlled trials (RCTs) and meta-analyses have established the efficacy of CPAP in reducing sleepiness and improving daytime functioning.8–11 Regarding cardiovascular outcomes, meta-analyses have demonstrated that CPAP reduces 24 h mean blood pressure (BP) by approximately 2 mm Hg (pooled estimated effect),12–17 the extent of improvement being larger in the subgroup with resistant hypertension.18 The intention-to-treat (ITT) analysis of the largest RCT conducted to date failed to show any significant effect of CPAP on incident hypertension and cardiovascular events, but showed a significant effect for the subgroup of patients with OSA using CPAP for more than 4 h a day.19 There is high heterogeneity in BP responses to CPAP, depending on OSA severity, nightly use of CPAP,20 ,21 and hypertensive status. Among factors influencing BP response to CPAP, there is little data regarding the role of CPAP treatment modalities; only a small sample-sized RCT suggests that fixed-pressure CPAP (FP-CPAP) is superior to auto-adjusting CPAP (AutoCPAP) vis-à-vis improvement in office BP.22
AutoCPAP changes the pressure delivered depending on the residual events detected at any given time, and applies the lowest effective pressure.23 The average overnight applied pressure is significantly lower with AutoCPAP, while comparably low apnoea+hypopnoea indices are achieved. Theoretically, when using AutoCPAP for long-term treatment, varying the pressure delivered would improve patient comfort and thus enhance compliance. However, the variations in pressure associated with the functioning of AutoCPAP devices may induce micro-arousals and potentially change sleep macrostructure in some patients.24 ,25 This could lead to greater sympathetic activity that counters BP reduction in response to CPAP treatment.
Thus, while AutoCPAP devices are increasingly used26 for long-term treatment of OSA, the reduction in BP remains a key issue for improving cardiovascular risk in patients with OSA. A clear answer regarding the respective impact of FP-CPAP versus AutoCPAP on BP is needed. The objective of our study was to conduct a large RCT to compare the efficacy of FP-CPAP with AutoCPAP in reducing BP in patients with OSA.
Methods
Design overview
The AgirSASadom study was a single-site randomised double-blind, parallel, 4-month controlled trial. The study was designed to assess whether FP-CPAP was superior to AutoCPAP in reducing BP in newly diagnosed patients with OSA. The trial was approved by the appropriate ethical committee, Comité de Protection des Personnes Sud-Est V, Grenoble, FRANCE (REC No: 09-AGIR-2) and registered on clinicaltrials.com (NCT01090297). All participants provided signed informed consent to participate in the study.
Participants
Patients referred to sleep clinics, usually due to daytime sleepiness, snoring, witnessed apneas or nocturia were assessed for eligibility. The inclusion criteria were patients with OSA aged 18–80 years with a recommendation to commence CPAP therapy. The exclusion criteria were pregnancy, cardiac failure and central sleep apnoea defined by a central apnoea index above 5/h. Patients were recruited from June 2010 and follow-up completed by October 2012. We should report one serious unintended event in one patient in the FP-CPAP group who presented a stroke during the protocol.
Procedures
Sleep studies
All patients were diagnosed with OSA using overnight respiratory polygraphy (PG) or polysomnography (PSG).
For polysomnography, continuous recordings were taken with electrode positions C3/A2-C4/A1-Cz/01 of the international 10–20 Electrode Placement System, eye movements, chin electromyogram and ECG with a modified V2 lead. Sleep was scored manually according to standard criteria.27 Cardiorespiratory polygraphy included at least the following signals: nasal pressure, thoracic and abdominal movements, SaO2, and body position. For polysomnography and polygraphy, airflow was measured with nasal pressure prongs, together with the sum of oral and nasal thermistor signals. Respiratory effort was monitored using abdominal and thoracic bands. Oxygen saturation was measured using a pulse oximeter. An apnoea was defined as the complete cessation of airflow for at least 10 s and hypopnoea as a reduction of at least 50% in the nasal pressure signal or a decrease of between 30% and 50% associated with either oxygen desaturation of at least 3% or an EEG arousal,27 both lasting for at least 10 s. Apnoea was classified as obstructive, central or mixed, according to the presence or absence of respiratory efforts. The classification of hypopnoea as obstructive or central was based on the thoracoabdominal band signal and the shape of the respiratory nasal pressure curve (flow limited aspect or not). The apnoea+hypopnoea index (AHI), defined as the number of apnoea and hypopnoea per hour of sleep (full polysomnography) or per hour of recording (polygraphy without EEG recording), was calculated. One hundred and twenty-seven patients had full PSG and 195 had respiratory polygraphy. The patients with PSG/PG were homogeneously distributed between the two arms, as expected for a large RCT (χ²: p=0.57).
BP measurements
Clinical BP was measured by mercury sphygmomanometer on three occasions in line with the European Society of Hypertension–European Society of Cardiology guidelines.28 Systolic BP (SBP) and diastolic BP (DBP) were assessed. Mean arterial BP (MABP) was calculated as DBP+1/3(SBP−DBP).
Ambulatory blood pressure monitoring (ABPM) was carried out with Spacelabs 90207 devices (Spacelabs International, Redmond, Washington, USA). The measurements were made every 15 min in the day and every 30 min at night. The following ABPM parameters were studied: mean SBP, DBP and HR over 24 h and during daytime (07:00–22:00) and nighttime (22:00–07:00). The summary values in the ABPM report for each patient in the data analysis were used.18 This was an average taken by subject and recording session (baseline/4 months). Data relating to the average daytime and nighttime SBP, DBP and mean BP were recorded. Values of SBP >260 mm Hg or <70 mm Hg and DBP >150 mm Hg or <40 mm Hg were automatically eliminated. Daytime hypertension was defined as daytime SBP >135 mm Hg and/or DBP >85 mm Hg, and nighttime hypertension as nighttime SBP >120 mm Hg and/or DBP >70 mm Hg.28
Carotid–femoral pulse wave velocity measurements
A one-pulse transducer was placed on the skin over the right common carotid and common femoral artery, with the patient in a supine position, and after at least 15 min of rest. The time delay of simultaneously recorded pulse waves between the carotid and the thigh was measured with a Complior device (Artech Medical; Pantin, France) and averaged over 10 consecutive cycles. The carotid–femoral pulse wave velocity (PWV) was calculated as the distance between the arterial sites divided by the time delay. The mean value of three consecutive measurements in each subject was used for analysis.
Randomisation and interventions
Patients meeting eligibility criteria were randomised to either FP-CPAP or AutoCPAP treatment. Randomisation was conducted by telephone call to a clinical trials statistician, independent of the study using a computer-generated random numbers list (six patients per block). In both arms, patients were randomly allocated to three different brands of the devices (Philips, Resmed and Weinman). Optimal CPAP pressure was titrated during eight nights at home using an AutoCPAP device (RESMED) to obtain a FP-CPAP value. The optimal pressure (95th percentile) was determined by one expert researcher, based on visual evaluation of the raw data recordings from nights with no significant leaks (less than 0.40 L/s). The mean CPAP pressure in the FP-CPAP arm was 9.3 mm Hg (SD: 1.9 mm Hg; median: 9.0 mm Hg). This fixed pressure was then maintained throughout the study in patients assigned to the FP-CPAP group. In the AutoCPAP group, the window for pressure variation was a minimum interval of 5 cm H2O. All patients, investigators and outcome assessment technicians were blinded to the arms to which the patients were allocated.
Main outcome measures
All outcome measures were made on two occasions, at baseline prior to treatment and then at the end of the 4 months’ treatment. The primary outcome was the change in office SBP between baseline and 4 months of FP-CPAP or AutoCPAP. Secondary cardiovascular outcomes included change in 24 h ambulatory BP and arterial stiffness.29
Statistical analysis
Continuous variables are expressed as median (25th/75th percentiles) or mean (SD), while categorical variables are reported as absolute numbers and percentages. Baseline comparisons between groups were made using Student's t test or Mann–Whitney U test, depending on the validation of normal distribution. For discrete variables, a χ2 test was used. Normality was assessed using the Shapiro–Wilk test. All randomised patients were included in the ITT analysis. The per-protocol (PP) population was defined as patients who completed the fourth month visit without any protocol deviation.
We powered the study based on an office BP outcome. A previous study showed that OSA treatment with FP-CPAP lowered systolic BP by 6 mm Hg relative to AutoCPAP.22 We assumed the impact of CPAP to be 6 mm Hg22 in the FP-CPAP group and 0 mm Hg in the AutoCPAP group (SD 15). Assuming an α error of 5% and a statistical power of 90%, 161 patients per arm needed to be enrolled in the study. An interim analysis was carried out when 150 patients had completed the study. The Peto group's sequential stopping method was used to preserve the intended α level and power by adopting stringent criteria (low nominal p values (p<0.001)) during the interim analyses.30 The interim analysis was defined a priori, validated by the ethics committee and included in the registration of the clinical trial. The intragroup differences from the beginning to the end of the study were evaluated with a paired t test. Unadjusted treatment effect and baseline BP adjusted treatment effect were estimated respectively by a mixed model with two factors (fixed factor: group; random factor: time) and by analysis of variance including baseline BP. In ITT analysis, missing data were replaced by multiple imputations using a regression model. We used a multiple imputation procedure that creates multiple imputed data sets with a regression method.31 Sequential regression models (from the least missing to the most missing variable) were created. This method assumes that the data are from a multivariate normal distribution; thus, when normality was not observed, variables were log-transformed before the imputation process and then were reverse-transformed to create the imputed data set. To guarantee the general validity of analyses of the resultant multiple imputed data set, we included as many variables as we could in the regression models.32
We had no missing data for clinical BP at baseline. For multiple imputations of BP at 4 months, we included baseline values of office BP, anthropometric parameters (age, body mass index, sex and waist circumference), medications, biological parameters, additional BP values, sleep apnoea parameters at baseline and under-treatment. Missing data for 24 h BP measurements have been imputed using the same methodology and by adding clinical BP values.
Data management and statistical analyses were performed using SAS (V.9.2, OSA Institute, Cary, North Carolina, USA).
Results
Of the initial 569 recruited patients, 322 were randomised, 161 to the FP-CPAP group and 161 to AutoCPAP treatment (ITT population) (figure 1). The middle-aged, obese, predominantly male and hypertensive study population reflected patients with OSA, usually seen in clinical practice (table 1). For the whole study population (table 2), the median (25th/75th percentiles) baseline 24 h mean BP was 94.0 (89.0/99.3) mm Hg; SBP, 128.0 (120.0/136.0) mm Hg and DBP, 78.0 (73.0/83.0) mm Hg. CPAP compliance did not differ between the groups with median (25th/75th percentiles) CPAP use of 5.2 (3.4/6.5) and 5.1 (3.5/6.6) hours per night in the FP-CPAP and AutoCPAP arms, respectively. The percentage of patients with OSA using CPAP more than 4 h/night was 64.6% and 66.5% in the FP-CPAP and AutoCPAP arms, respectively. The median (25th/75th percentiles) residual AHI following the application of CPAP was 3.6 (2.1/6.9) events/h without difference between groups (AutoCPAP: 3.6 (2.2/6.5) and FP-CPAP: 3.6 (2.0/7.9) respectively).
Baseline characteristics of all randomised patients
Baseline BP and arterial stiffness characteristics
Flow diagram showing the trial allocation.
ITT analysis
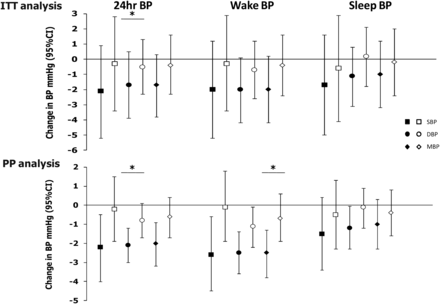
When the changes in BP from baseline to 4 months were compared between study groups by ITT, the office systolic BP decreased by 2.2 mm Hg (95% CI −5.8 to 1.4) in the FP-CPAP group and by 0.4 mm Hg (95% CI −4.3 to 3.4) in the AutoCPAP group (difference between groups: −1.3 mm Hg (95% CI −4.1 to 1.5); p=0.37, adjusted for baseline BP values and history of hypertension) (table 3). From baseline to 4 months, 24 h DBP decreased by 1.7 mm Hg (95% CI −3.9 to 0.5) in the FP-CPAP group and by 0.5 mm Hg (95% CI −2.3 to 1.3) in the AutoCPAP group (difference between groups: −1.4 mm Hg (95% CI −2.7 to −0.01); p=0.048, adjusted for baseline BP values and history of hypertension). In both arms, the prevalence of non-dipping status was unchanged after the intervention.
Effect of CPAP treatments on BP levels in the intention-to-treat population
PP analysis
In a PP analysis (133 patients in the FP-CPAP group; 143 patients in the AutoCPAP group), patients in the FP-CPAP group showed a statistically significant decrease of 1.3 mm Hg (95% CI 2.4 to 0.1), p=0.032 in 24 h DBP and 1.6 mm Hg (95% CI 3.1 to 0.01), p=0.049 in 24 h mean daytime BP (table 4 and figure 2). Graphically, it can be clearly seen (figure 2) that the range of decrease is greater with FP-CPAP when compared with AutoCPAP.
Effect of CPAP treatments on BP levels in the per-protocol population
Change from baseline in 24 h blood pressure variables. Data represent mean differences from baseline (95% CI) on FP-CPAP (closed symbols) and AutoCPAP (open symbols) for the 24 h, Wake and Sleep periods. Top panels: Intention-to-treat (ITT) analysis. Bottom panels: Per-protocol (PP) analysis. *p<0.05. BP, blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; SBP, systolic blood pressure.
A direct comparison of the two CPAP arms restricted to the subgroup of good compliers (>4 h/night) did not demonstrate a superiority of one CPAP modality, both in ITT and PP analyses. Improvement in quality of life as assessed by SF12 was the same in the two arms.
Discussion
Hypertension affects about 25% of the adult population worldwide. It ranks as the leading chronic risk factor for mortality, accounting for 13.5% of all deaths. Half of all strokes and ischaemic heart disease events are attributable to high BP.33 ,34 OSA is now recognised as a risk factor for the development of hypertension in European and US International Guidelines. After accounting for the main confounders, OSA and hypertension remain linked in a dose–response fashion.3 In a large prospective observational cohort followed for more than 12 years, it was shown that compared with controls, the adjusted HRs for incident hypertension were greater among patients with severe OSA who declined CPAP therapy and among those non-adherent to CPAP therapy, whereas the HR was lower in patients with OSA who were treated with CPAP therapy for more than 4 h/night.35 This suggests that treating OSA could be an important step towards reducing BP and thus cardiovascular risk. Meta-analyses of RCTs have demonstrated that CPAP reduces 24 h mean BP by approximately −2 mm Hg (pooled estimated effect).12–17 In these meta-analyses, heterogeneity is a clear concern. Also, Bratton et al20 found no beneficial effect on BP in patients with minimally symptomatic OSA, except in those patients who used CPAP for >4 h/night.21 In summary, meta-analyses agree on the fact that BP reduction after CPAP initiation is of greater amplitude in patients with severe OSA and/or poorly controlled/resistant hypertension18 and in patients who comply with CPAP treatment. Surprisingly, the impact of different CPAP modalities on BP has been little studied. Our study, comparing FP-CPAP with AutoCPAP, was negative regarding its primary outcome of office BP, while FP-CPAP was more effective in reducing 24 h DBP (a secondary outcome).
Three RCTs have assessed changes in BP induced by FP-CPAP versus AutoCPAP.22 ,36 ,37 Two studies did not find significant differences in BP changes between the two groups.36 ,38 ,39 In these two studies, BP was a secondary outcome and, as stated by the authors themselves, the studies were not powered to look at the changes in BP with CPAP treatment.38 In the third study,22 significant reductions in office BP were observed in the FP-CPAP group but not in the AutoCPAP group. Ip et al,39 using data reported by Patruno et al,22 estimated a nearly significant greater reduction in SBP (6 mm Hg (95% CI −1 to 13 mm Hg); p=0.09) and a significant greater reduction in DBP (8 mm Hg (95% CI 4 to 11 mm Hg); p<0.001) with FP-CPAP compared with AutoCPAP. We used office SBP as the outcome in our study as it was the only significant outcome in the Patruno study and has previously been frequently used in studies comparing CPAP with an inactive control (44 studies in the meta-analysis by Bratton et al17). This outcome is relevant as it is related to mortality and the incidence of late cardiovascular events.40 However, given the relatively small number of subjects in previous studies, these findings needed to be re-evaluated in a more highly powered study that also included 24 h BP monitoring. Our study was clearly negative regarding the primary outcome, and the difference between the two CPAP modalities (1.4 mm Hg (CI 95% 2.7 to 0.01)) might appear to be of limited clinical relevance. However, even small effects on BP in the range of 1–2 mm Hg are of clinical significance as they are associated with reduced odds of cardiovascular and cerebrovascular events.20 ,40 ,41 There is increasing evidence that mean nocturnal BP is a major indicator of cardiovascular morbidity and mortality, irrespective of clinical BP.42 The 24 h BP measurements are more sensitive to therapeutic interventions and provide specific prognostic information. This is particularly true in sleep apnoea-related hypertension.18 ,43 ,44 While negative in terms of the primary outcome, our study provides interesting insights regarding 24 h ABPM that might be of clinical relevance and a strong rationale for further studies.
One can hypothesise on the mechanisms potentially underlying the different impacts on BP of the two treatment modalities. Sleep fragmentation and alterations in sleep quality are associated with prevalent or incident hypertension, even if age, gender, environmental exposures and ethnic disparities are clear confounders.42 Any disturbance in sleep quality is a mechanism through which an increase in sympathetic tone and in turn elevated BP can appear. The manner in which AutoCPAP functions is associated with a more unstable upper airway that can sometimes induce micro-arousals.24 ,25 Also, inappropriate increases in pressure can occur even in normal subjects after a change in body position, after a central apnoea, during a period of flow limitation or, most frequently, without any identifiable reason.45 There are potentially differences from one brand of AutoCPAP device to another. Devices differ in how sleep-disturbed breathing is detected and in how the operational algorithm responds. Accordingly, one of the strengths of our study was to have randomised to three different brands in the AutoCPAP arm. Thus, our results are not related to a particular brand. As stated above, acute increases in pressure can induce micro-arousals from sleep that have been associated with autonomic activation. Karasulu et al46 demonstrated that AutoCPAP devices did not improve heart rate variability, whereas FP-CPAP did. Also, AutoCPAP treatment was characterised by greater sympathetic activation and was associated with poorer cardiorespiratory coupling compared with FP-CPAP.47 Although our study did not aim to address these specific mechanisms, these figures provide a clear rationale towards explaining our results.
Study limitations
We adopted a clinical approach to setting up CPAP therapy, without preliminary in-laboratory CPAP titration. This might be considered as a limitation, but it reflects clinical practice and has become standard in many European sleep centres.48 Moreover, neither the index of residual events nor compliance differed between the two arms. The expected decrease of 6 mm Hg in SBP in the fixed-pressure arm based on the preliminary data of Patruno et al22 was probably not realistic to achieve. The recent meta-analysis by Bratton et al17 shows that compared with an inactive control, CPAP was associated with a reduction in SBP of 2.5 mm Hg (95% CI 1.5 to 3.5 mm Hg). This is exactly the effect size that we found in our FP-CPAP arm.
Conclusions and perspectives
Generally, the impact of CPAP in reducing BP is relatively modest even in patients with sleepy OSA. Our study did not demonstrate that FP-CPAP is more effective than AutoCPAP regarding the primary outcome of office BP. However, indicating FP-CPAP treatment for patients with OSA with high cardiovascular risk, such as those with poorly controlled or resistant hypertension, might be of interest as our study found a higher impact of FP-CPAP than AutoCPAP, on 24 h DBP (a secondary outcome).
Acknowledgments
The authors thank Alison Foote (Grenoble Alpes University Hospital) for editing the language.
References
Footnotes
JLP and RT are co-first authors.
JFT and PL are co-senior authors.
Contributors JLP led the study and wrote the first draft of the report. NA and JFT undertook the data analyses. All authors participated in design, execution and oversight of the study. All authors had access to the data, commented on subsequent drafts and approved the final submitted version. JLP will act as guarantor and made the final decision to submit the manuscript for publication.
Funding The study was funded by the “Fondation Agir pour les maladies chroniques”. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Competing interests None declared.
Patient consent Obtained.
Ethics approval Comité de Protection des Personnes Sud-Est V.
Provenance and peer review Not commissioned; externally peer reviewed.