Article Text
Abstract
Objective Intranasal application of cowshed dust extract (CDE) during sensitisation in a murine model of experimental asthma leads to a significant alleviation of the clinical parameters of the allergic immune response. However, neither the immunological mechanisms underlying this protective effect nor all of the protective substances included in CDE have yet been described. Recently, complement factor 5a (C5a) receptor signalling has been identified to play a regulatory role in allergic airway disease. Thus we investigated whether CDE can activate the complement system to release biologically active C5a in the lung.
Methods Proteins included in CDE were identified by mass spectrometry. Complement cleaving activity of a serine protease identified in CDE was validated with the purified enzyme, and the biological activity of the released C5a was determined. C5a was applied in a murine model of allergy to prove its protective impact on allergic airway disease.
Results CDE induced the release of C5a in murine bronchoalveolar lavages (BAL). We identified a serine protease from the midgut of tenebrio molitor larvae in CDEs which was able to induce the release of biologically active C5a in murine BAL. We applied C5a in different doses to female Balb/c mice during the sensitisation phase and during the first antigen challenge and showed that C5a has the ability to dampen important parameters of allergic airway inflammation, such as infiltration of proinflammatory cells into lung tissue or Th2 cytokine secretion by lung cells.
Conclusions We conclude that the C5a generating enzyme included in CDE might account for some of the allergy protective effects of CDE by generation of C5a in murine lungs.
- Allergic lung disease
Statistics from Altmetric.com
Key messages
What is the key question?
-
Which substances of the farming environment are responsible for allergy protection, and what type of immunological mechanisms are involved in protection?
What is the bottom line?
-
A serine protease is included in cowshed dust extracts which induces the release of biologically active C5a in murine bronchoalveolar lavage fluids. C5a itself has a protective impact on allergic airway disease in a murine model of experimental asthma.
Why read on?
-
Prevalence and incidence of allergic diseases are still increasing. Immunomodulation could be a promising approach for the prevention of allergic diseases.
Background
The hygiene hypothesis explains the increasing prevalence and incidence of allergic disorders during the past few decades by a decreased infection rate of children in their early childhood.1 It has been repeatedly demonstrated that children who grow up on traditional farms are protected from allergic diseases such as asthma and hay fever.2–5 There is an association between contact with cowsheds in the first year of life and protection against allergic disorders, suggesting that immune modulation is mediated by inhalation of cowshed dust extract (CDE). As a proof of principle, mice were sensitised intraperitoneally with ovalbumin adsorbed to aluminum hydroxide (OVA/Alum) and CDE was simultaneously administered intranasally during the sensitisation phase. This treatment leads to alleviation of many important parameters of allergic asthma.6 It is of great interest to identify the substances and the immunological mechanisms that are responsible for the protective effect. In addition to two protective bacterial strains in cowshed dust,7 we recently identified arabinogalactans (AG) as a protective group of sugar molecules included in CDE.8 These sugar molecules modulate the function of dendritic cells, which are then not able to induce allergic sensitisation and airway inflammation. Polysaccharides, such as AG, are known to activate the complement system9 and, importantly, it has been shown that activation of the complement system plays a dual role in the development of allergic immune responses. Depending on the time point of activation, the complement factor 5a receptor (C5aR) can either act proinflammatorily or in a regulatory way.10–12 The complement system can be activated in three different ways—that is, the classical, alternative or mannose binding lectin way—but also by proteases not belonging to the complement system which can directly cleave complement associated zymogens.13 ,14 We therefore examined the dust extracts for complement activating properties. Surprisingly, we found a serine protease from tenebrio molitor larvae to be responsible for the CDE induced complement factor 5a (C5a) release in murine bronchoalveolar lavage (BAL) fluids. This protease cleaves C5 and releases biologically active C5a. We hypothesised that the release of C5a by the protease may be one possible immunological mechanism which is responsible for the protective effect of CDE. As a proof of principle, we administered recombinant C5a (rC5a) intranasally to mice during the sensitisation phase and during the first antigen challenge to determine whether C5a has a relevant impact on the development of allergic airway inflammation. We showed that treatment with rC5a in our mouse model leads to reduction of infiltrating leucocytes into lung tissue, lower systemic IgE titres and a reduced Th2 cytokine production of lung cells on antigen recall. We conclude that the release of C5a by protease is one of the immunological mechanisms underlying the protective effect of CDE.
Material and methods
Extraction of cowshed dust
Collection of dust from 30 farming households in the region of the rural Alps and extraction of dust was performed as described previously.6
Chromatography of CDE
Size exclusion chromatography on Superdex G200 was performed using an Amersham XK 16/60 column: 1 ml of a 50 mg/ml CDE solution was separated with a linear flow rate of 1 ml/min. Those samples with highest proteolytic activity were combined and concentrated to a final volume of 1 ml. Anion exchange was performed on MonoQ Gl 5/50 (GE Healthcare, Munich, Germany). Each sample was tested for proteolytic activity.
S2288 protease assay
Chromogenix S2288 substrate (Haemachrom Diagnostica GmbH, Essen, Germany) was used to quantify the proteolytic activity of serine proteases. Proteolytic activity was measured as described by the manufacturer.
Zymography
Sample (15 µl) was loaded onto 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) copolymerised with 1% bovine serum albumin. After removing SDS and incubating overnight, the gel was stained with Coomassie brilliant Blue R250 and destained until bands of proteolysis were visible. Zones of distinct proteolytic activity were excised and used for mass spectrometric analysis.
Liquid chromatography and mass spectrometry analysis
Tryptic in-gel digest and analysis of the peptides with an Ultimate nanoflow HPLC system (Dionex/LC Packings) coupled to a QSTAR Pulsar i Hybrid QqTOF mass spectrometer (Applied Biosystems/MDS Sciex) equipped with a nanoelectrospray ion source were carried out as described previously.15
Incubation of murine BAL fluids with CDE or purified TmT1
Collection of BALs from sensitised and challenged mice was performed as described previously.6 Bronchoalveolar lavage fluid (BALF 150 μl) was incubated with 10 μl of CDE (5 mg/ml) or TmT1 (0.029 U) for 30 min at 37°C. The reaction was stopped by adding 1 μl of aprotinin (50 mg/ml). Solutions were stored at −20°C until further analysis.
Determination of C5a in murine BALFs by ELISA
C5a in murine BAL was determined by sandwich ELISA. Recombinant murine C5a (Hycultec GmbH, Beutelsbach, Germany) was used as a protein standard. Nunc Maxisorp plates (Fisher Scientific, Schwerte, Germany) were coated with purified rat antimouse C5a (BD, New Jersey, USA) in carbonate buffer (8.4 g/l NaHCO3, 3.56 g/l Na2CO3, pH 9.5). Free binding sites were blocked by incubating the plates in phosphate buffered saline–10% fetal calf serum. Rat biotin antimouse C5a (BD) was used as a second antibody and extravidin peroxidase (Thermofisher Scientific, Waltham, Massachusetts, USA) and 3,3′,5,5′-tetramethylbenzidine substrate kit for detection.
Preparation of TmT1 serine protease from the midgut of tenebrio molitor larvae
The posterior midgut from tenebrio molitor larvae was prepared and protease was isolated, as described previously.16
Expression of rC5a in E Coli Bl21(pLysS)
E Coli BL21(pLysS) cells containing the pET15b vector with murine C5a insert (a kind gift from P Ward, Michigan, USA) were used for the recombinant expression of C5a. rC5a was isolated by affinity chromatography using HisPur cobalt columns (Thermo Scientific, Rockford, Illinois, USA), as described by the manufacturer. We used Endotrap blue columns (Hyglos, Bernried, Germany), as described by the manufacturer, to remove endotoxin contaminations (resulting in 10 ng lipopolysaccharide per 1 mg rC5a).
Chemotaxis assay with murine cell line J774A.1
A chemotaxis assay using the Fluoroblok 24-Multiwell Insert System (BD) and J774A.1 cells was performed to determine the biological activity of C5a. In some experimental approaches, anti-C5a receptor monoclonal antibody 20/70 (Hycultec GmbH, Beutelsbach, Germany) or an IgG2b isotype control antibody (Immunotools, Frisoythe, Germany) was added to a final concentration of 10 μg/ml to the cells. After cell migration, the inserts were washed in RPMI for 30 min at 7°C and incubated with Calcein-AM (Merck, Darmstadt, Germany) for 30 min at 37°C, 5% CO2. Afterwards, the membranes were washed in RPMI medium and cells that migrated to the lower side of the membrane were counted by fluorescence microscopy.
Sensitisation, treatment and airway challenge of animals
All experimental procedures were approved by the animal ethics committee at Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, Germany. Sensitisation, challenge and treatment of the 7–8 week old female Balb/c mice were performed according to a scheme described previously.6 A detailed protocol can be found in the online data supplement (see supplementary figure S1, available online only). The lungs were prepared and fixed in formalin for histology, as described elsewhere.8 In vitro cultures of lung cells were prepared as described elsewhere.6 Lung cell suspensions were stimulated with 50 µg of ovalbumin for 48 h and supernatants were collected for cytokine determination by ELISA.
Enzyme linked immunosorbent assay (ELISA)
BD OptEIA Kits (BD Pharmingen) were used as described by the manufacturer for the determination of interleukin (IL)-4, IL-5, IL-10 and IL-13 in cell culture supernatants. Determination of OVA specific IgE titres in serum and BALFs was performed as described previously.6
Statistical analysis
All data were analysed by one way ANOVA Kruskal–Wallis test and subsequent Dunns test. Treated groups were compared with the untreated group using Graph Pad Prism software V.5 (La Jolla, California, USA) for analysis. Values of p<0.05 were considered statistically significant (*p<0.05, **p<0.01, ***p<0.001). Results are presented as median or mean±SD, as indicated.
Results
A protease from CDE induces the release of C5a in murine BALFs
We first investigated whether CDE was able to activate the complement system in murine BALFs. We focused on the release of C5a, as the receptor of this anaphylatoxin has been described as playing a regulatory role during the sensitisation phase in a murine model of allergy. Incubation of BALFs with CDE led to a dose dependent release of C5a (figure 1). The identity of C5a was confirmed by western blot analysis (see supplementary figure S2, available online only). Importantly, the release of C5a was significantly inhibited if CDE was preincubated with aprotinin, revealing that a serine protease is responsible for cleaving C5 in BALF. Furthermore, we observed only one pH and one temperature optimum for proteolytic activity in CDE (see supplementary figure S3, available online only). This let us to hypothesise that the C5a release in murine BALFs was mainly caused by the activity of a single serine protease present in CDE.
Complement factor 5a (C5a) release in murine bronchoalveolar lavage fluids (BALFs) by cowshed dust extract (CDE): 150 μl of murine BALFs were incubated with 10 μl of dust extract, resulting in different amounts of proteolytic activity in the approaches (from 0.18 U/ml to 0.009 U/ml) or with dust extract coincubated with aprotinin (aprot.) to inhibit proteolytic activity. Three independent experiments were performed with similar results. n=6 in each group. *p<0.05, **p<0.01. PBS, phosphate buffered saline.
Isolation and identification of the protease from CDE
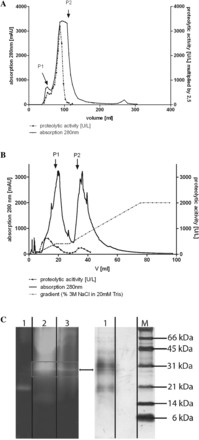
Next we sought to isolate and identify the protease from dust extract. Applying CDE to a Superdex G200 column resulted in two elution maxima (P1 and P2, figure 2A). Those fractions containing the most proteolytic activity were applied to an anion exchange column. The elution profile yielded two elution maxima (P1 and P2, figure 2B). The fractions containing the most proteolytic activity were loaded onto 15% SDS-PAGE and zymography was performed (figure 2C). The band on SDS-PAGE that showed proteolytic activity in zymography (indicated by an arrow and box in figure 2C) was excised and used for mass spectrometry. The protease was identified as posterior midgut digestive trypsin (namely, TmT1) from tenebrio molitor larvae (see supplementary table S1, available online only).
Isolation and identification of the serine protease from cowshed dust extract (CDE). (A) Size exclusion chromatography on Superdex G200 Amersham XK 16/60 column. CDE (50 mg/ml) was dissolved in 1 ml of sterile aqueous 0.9% NaCl solution and applied to the column, the run was performed with 1 ml/min and samples were collected in 5 ml steps. Proteolytic activity was determined by S2288 protease assay. (B) Anion exchange chromatography on a MonoQ GL 5/50 column. Fractions containing the highest proteolytic activity were concentrated to 1 ml and loaded onto an anion exchange column in 20 mM Tris and 10 mM NaCl. The run was performed with 0.5 ml/min and samples were collected in 2 ml steps. Absorption was measured at 280 nm while proteolytic activity was determined by S2288 protease assay. (C) Fractions from anion exchange chromatography containing the highest proteolytic activity were loaded onto 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (right, lane 1) and zymography was performed (left, lane 3). The proteolytic active band (indicated by the box) was excised and used for mass spectrometry. Zymography: 1=trypsin positive control, 2=crude CDE and 3=fractions 8 and 9 from anion exchange chromatography. The experiment was performed twice. This figure is only reproduced in colour in the online version.
Release of biologically active C5a in murine BALFs by isolated TmT1 serine protease
We sought to determine whether purified TmT1 was able to induce the release of C5a in murine BALFs. To isolate the protease, we performed anion exchange chromatography with an extract of the midgut contents. Adjusting the pH of the running buffer to the isoelectric point of the protease (approximately 8.5) led to rapid elution of the protease from the column (figure 3A, fraction F3). The purity of the enzyme was confirmed by SDS-PAGE and silver staining (figure 3B), whereas the identity was confirmed by mass spectrometry (see supplementary table S2, available online only). Zymography confirmed the proteolytic activity of the enzyme (see supplementary figure S4, available online only). Incubation of murine BALFs with the purified enzyme led to the release of C5a, which was dependent on the dose of the protease. Inhibiting proteolytic activity by heat denaturation resulted in a decrease in C5a release to a level that was detectable in the negative control (phosphate buffered saline), showing that proteolytic activity of TmT1 was responsible for C5a release (figure 3C).
Isolation of TmT1 protease and TmT1 mediated complement factor 5a (C5a) release in murine bronchoalveolar lavage fluids (BALFs). (A) Anion exchange chromatography with extracts from the midgut of the tenebrio molitor larvae. Peak 2 (P2) contained TmT1 protease, as shown in (B). (B) Silver stained 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis of peak 2 from anion exchange chromatography. (C) Purified TmT1 protease from anion exchange chromatography was incubated in different amounts with murine BALFs for 30 min. Afterwards, C5a release was determined by ELISA. n=2 per group. The experiment was performed twice with similar results. PBS, phosphate buffered saline.
We performed a chemotaxis assay using the murine macrophage cell line J774A.1. BALFs were incubated with native TmT1, heat denatured TmT1 or RPMI medium as a negative control. J774A.1 cells migrated through the pores if samples that contained TmT1 released C5a were applied to the lower chambers (figure 4). No migration was observed if samples that had been incubated with heat denatured TmT1 (no C5a release) were applied to the lower chambers. Cells that were treated with the C5aR neutralising antibody did not migrate if BALFs containing TmT1 released C5a were loaded into the lower chambers, showing that TmT1 protease has the ability to induce the release of biologically active C5a in murine BALFs. Interestingly, we observed that incubation of human C5 with TmT1 protease led to the cleavage of C5-α chain and the release of C5a, indicating that C5a generation in BALFs is due to direct cleavage of C5 by TmT1 (see supplementary figure S5, available online only).
Determination of the biological activity of complement factor 5a (C5a). Chemotactic activity of murine J774A.1 cell line in BD Fluoroblok chemotaxis assay. Cells were incubated with bronchoalveolar lavage fluids (BALFs) incubated with native TmT1 (BALF+0.18 U/ml TmT1, ie, 130.6±7.9 ng/ml C5a in lower chamber, n=5) or with BALFs incubated with heat denatured TmT1 (BALF+0.18 U/ml TmT1heat denatured, ie, approximately 15 ng/ml C5a in the lower chamber, n=3). In another approach, cells were preincubated with antimurine C5aR antibody (BALF+0.18 U/ml TmT1+anti-CD88, n=5) or rat antimouse IgG2b isotype control (BALF+0.18 U/ml TmT1+isotype control, n=2). Both antibodies were used in a concentration of 10 µg/ml. Results are presented as percentage of positive control (850 ng/ml rC5a in lower chamber). Results are presented as mean±SEM.
Application of rC5a in vivo alleviates important parameters of allergic airway inflammation
Next we sought to determine if the application of rC5a intranasally to mice during the sensitisation phase and during the first antigen challenge (see supplementary figure S1, available online only) had an impact on the development of the allergic phenotype. We observed a significant reduction in the numbers of leucocytes which infiltrated into the lungs of animals that received 10 µg C5a per treatment compared with animals that were sham treated with 0.9% NaCl (figure 5A). This reduction was due to a significant reduction in eosinophils and lymphocytes (figure 5B). We did not observe any differences in the airway reactivity to methacholine between sham treated mice and mice that were treated with 10 µg C5a (see supplementary figure S6, available online only). Lung cells of mice that were treated with 10 µg C5a secreted significantly less IL-5 and IL-13 compared with lung cells of animals that were sham treated (figure 6A, B) whereas IL-4 and IL-10 were also reduced, but these differences were not significant (figure 6C, D). We observed a non-significant reduction in systemic IgE titres on treatment with 10 or 2 µg C5a (figure 7). Lung histology showed that infiltration of leucocytes into lung tissue was reduced on treatment of mice with 10 or 2 µg rC5a. However, treatment with 0.2 µg rC5a was not sufficient to induce such a strong reduction (figure 8). Additionally, the goblet cell hyperplasia was not abolished by treatment of mice with rC5a (figure 8).
Cellular parameters of allergic airway inflammation. (A) Number of leucocytes in the bronchoalveolar lavage fluids (BALFs) of ovalbumin (OVA) sensitised and OVA challenged mice (NaCl, 10 µg complement factor 5a (C5a), 2 µg C5a, 0.2 µg C5a, n=6 in all groups) and number of leucocytes in the BALFs of sham sensitised and OVA challenged mice (not sensitised (n.s.)+10 µg C5a and n.s.+NaCl, n=4 in both groups). Mice were treated with rC5a or sham treated with NaCl during the sensitisation and around the first antigen challenge, as indicated in supplementary figure S1 (available online only). (B) Number of eosinophilic granulocytes, macrophages, neutrophilic granulocytes and lymphocytes in BALFs of mice of differently treated groups. *p<0.05, **p<0.01, as determined by the ANOVA Kruskal–Wallis-test. Results are presented as mean±SEM. The experiment was performed twice with similar results for groups NaCl, 10 µg C5a, 2 µg C5a and 0.2 µg C5a. The experiment was performed once for control groups n.s.+10 µg C5a and n.s.+NaCl.
Amount of cytokines in supernatants of ovalbumin stimulated lung cells. (A) Interleukin (IL)-5, (B) IL-13, (C) IL-4 and (D) IL-10. n=4 in all groups *p<0.05, as determined by the ANOVA Kruskal–Wallis test. Results are presented as mean±SEM.
Amounts of ovalbumin (OVA) specific IgE levels in serum. OVA specific IgE titres in the serum of differently treated mice were determined by ELISA. Results are presented as mean±SEM. n=6 in all groups. n.s., not sensitised.
Lung histology. Haematoxylin–eosin (H&E) staining and periodic acid–Schiff (PAS) staining of representative 1 µm lung slices of mice in the differently treated groups. Left panel: magnification=10-fold; middle panel: magnification=40-fold; right panel: magnification=10-fold. NaCl=mice sensitised to ovalbumin/aluminum hydroxide (OVA/Alum), treated with NaCl and challenged with ovalbumin; 10 µg/2 µg /0.2 µg complement factor 5a (C5a) =mice sensitised to ovalbumin (OVA/Alum), treated with 10 µg, 2 µg or 0.2 µg C5a and challenged with ovalbumin; not sensitised+10 µg C5a=mice sham sensitised with phosphate buffered saline (PBS)/Alum, treated with 10 µg C5a and challenged with ovalbumin; not sensitised+NaCl=mice sham sensitised with PBS/Alum, treated with NaCl and challenged with ovalbumin. This figure is only reproduced in colour in the online version.
Discussion
In a previous study, we showed that inhalative treatment with CDE during sensitisation in a murine model of allergy leads to protection from allergic disease.6 We have identified AG as one important class of substances responsible for the protective effect of CDE.8 However, there are still immunomodulatory substances detectable in CDE. As polysaccharides are known to activate the complement system, and C5aR signalling during the sensitisation phase has a protective effect on the development of important parameters of allergic asthma,10 ,11 ,17 we investigated CDE for complement activating properties in this study. We showed that CDE induced the release of C5a in murine BALFs which was mainly caused by a serine protease included in CDE, whereas AG did not induce the release of C5a (data not shown). The protease was identified by mass spectrometry as TmT1 posterior midgut digestive trypsin, an enzyme derived from tenebrio molitor larvae which is known as a stored product pest that lives on traditional farms. We isolated the protease from the midgut of tenebrio molitor larvae and showed that purified TmT1 had the ability to induce the release of biologically active C5a in murine BALFs. This result lets us conclude that TmT1 is able to perform a limited proteolysis of C5 and that TmT1 has properties similar to the C5 convertase, a feature which is not common to serine proteases but has also been described for some other serine proteases.13 ,14 This is even more important as we showed that TmT1 can directly cleave human C5 leading to release of C5a (see supplementary figure S4, available online only). Therefore, it is tempting to speculate that C5a is probably also generated in the lungs of children inhaling dust of cowsheds. There is strong evidence that C5a is released by TmT1 in the lungs of mice which have been treated with CDE to protect them from allergic asthma. The proteolytic activity of allergens can enhance or augment allergic sensitisation and airway inflammation in murine models if the allergen is applied through the pulmonary route for sensitisation.18–21 Serine proteases are also known to cause tissue damage by degradation of tight junctions.22 ,23 Furthermore, some proteolytic active allergens are known to activate protease activated receptor 2,24 ,25 which either enhances allergic sensitisation and inflammation26 or has a protective impact on allergic airway inflammation.27 Thus to avoid bystander effects caused by the proteolytic activity of TmT1 and to enlighten the relevance of C5a occurrence during sensitisation for protection in our mouse model, we applied rC5a instead of purified TmT1 protease to the mice during the sensitisation phase and during the first antigen challenge. We showed that application of rC5a dose dependently led to a reduction in important parameters of allergic airway inflammation. We present strong evidence that the allergy protective properties of CDE can, at least partly, be explained by the TmT1 induced occurrence of C5a during the sensitisation phase and during the first antigen challenge. This is true because we could clearly show that the occurrence of C5a during this period has a relevant impact on the allergic phenotype in our mouse model, and that CDE has the ability to release C5a by the proteolytic activity of TmT1. It has not yet been shown that direct application of rC5a during the sensitisation phase and during first antigen challenge can alleviate the symptoms of allergic airway inflammation. Others used either C5a receptor blocking antibodies or C5a receptor knockout mice to show that C5aR signalling is critically involved in the downregulation of important parameters of allergic airway disease. However, in our experiments, we titrated rC5a from 10 to 0.2 µg per application as other substances, such as lipopolysaccharide or other immunomodulatory substances, have also been intranasally applied in these ranges to prove their impact on the allergic phenotype.28 Importantly, in our approach, we gave short stimuli to the C5a receptor by the intranasal instillation of rC5a rather than a continuous stimulus to C5aR. These short stimuli for a total of 14 times during sensitisation were sufficient to dampen clinically relevant parameters of allergic airway inflammation. It is unclear, at this point, if continuous stimulation of the C5aR by C5a during the sensitisation phase and during first antigen challenge would lead to a more robust alleviation of the allergic phenotype. It is also unclear whether smaller amounts of C5a would be sufficient to alleviate symptoms of allergic airway inflammation if a continuous release of C5a into lung tissue could be achieved (eg, by encapsulating C5a into liposomes). Although others observed that ablation of C5aR signalling during sensitisation led to an increased airway hyper-responsiveness (AHR), we did not observe alleviation of AHR on treatment of mice with C5a (see supplementary figure S6, available online only). This is in accordance with our observations that rC5a treatment did not abolish goblet cell hyperplasia (figure 8). However, we observed a significant reduction in the secretion of IL-13 by OVA restimulated lung cells after treatment of mice with 10 µg rC5a. IL-13 has been described as an important cytokine for eosinophil recruitment and survival,29 which is in accordance with our observations of reduced eosinophil recruitment into lung tissue. However, IL-13 is also known to be an important inducer of AHR.30 Although IL-13 was significantly reduced in cell culture supernatants of OVA restimulated lung cells of mice treated with 10 µg rC5a, we did not observe a reduction in AHR. The investigation of this phenomenon will be part of future studies.
We have shown in this study that a serine protease from the midgut of the tenebrio molitor larvae is included in CDEs and has the ability to release biologically active C5a in murine BALFs. We have shown that activation of the complement system during the sensitisation phase and during first antigen challenge in our model of allergy has a relevant impact on the development of the allergic phenotype. We therefore conclude that the C5a release caused by proteolytic activity in CDE may be one relevant piece of the puzzle of immunological mechanisms underlying the protective effects of CDE.
Acknowledgments
We would like to thank Sandra Busse, Petra Fritz and Britta Steeger at Bochum, and Petra Behrens at Forschungszentrum Borstel (Borstel, Germany), for excellent technical assistance, and Johannes Madlung at Proteom Centre Tübingen for the mass spectrometric analysis. We also thank Stephanie Neuhaus and Imke Steffen for technical assistance. We thank Philip Saunders (Language Support Services, Berlin, Germany) for proofreading the manuscript. Furthermore, we would like to thank Benjamin Fränzel and Dirk Wolters at Ruhr-University Bochum for mass spectrometric analysis of human C5a.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online supplement
Footnotes
-
Contributors MS conducted the study, analysed the data and drafted the manuscript. AB discussed the data and revised the manuscript. MP initiated and planned the study, discussed the data and revised the manuscript.
-
Competing interests None.
-
Ethics approval Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, Germany.
-
Provenance and peer review Not commissioned; externally peer reviewed.