Article Text
Abstract
Background: Intrinsic positive end expiratory pressure (PEEPi) constitutes an inspiratory threshold load on the respiratory muscles, increasing work of breathing. The role of continuous positive airway pressure (CPAP) in alleviating PEEPi in patients with severe stable chronic obstructive pulmonary disease is uncertain. This study examined the effect of CPAP on the inspiratory threshold load, muscle effort, and lung volume in this patient group.
Methods: Nine patients were studied at baseline and with CPAP increasing in increments of 1 cm H2O to a maximum of 10 cm H2O. Breathing pattern and minute ventilation (V̇i), dynamic PEEPi, expiratory muscle activity, diaphragmatic (PTPdi/min) and oesophageal (PTPoes/min) pressure-time product per minute, integrated diaphragmatic (EMGdi) and intercostal EMG (EMGic) and end expiratory lung volume (EELV) were measured.
Results: Expiratory muscle activity was present at baseline in one subject. In the remaining eight, PEEPi was reduced from a mean (SE) of 2.9 (0.6) cm H2O to 0.9 (0.1) cm H2O (p<0.05). In two subjects expiratory muscle activity contributed to PEEPi at higher pressures. There were no changes in respiratory pattern but V̇i increased from 9.2 (0.6) l/min to 10.7 (1.1) l/min (p<0.05). EMGdi remained stable while EMGic increased significantly. PTPoes/min decreased, although this did not reach statistical significance. PTPdi/min decreased significantly from 242.1 (32.1) cm H2O.s/min to 112.9 (21.7) cm H2O.s/min). EELV increased by 1.1 (0.3) l (p<0.01).
Conclusion: High levels of CPAP reduce PEEPi and indices of muscle effort in patients with severe stable COPD, but only at the expense of substantial increases in lung volume.
- chronic obstructive pulmonary disease
- continuous positive airway pressure
- positive end expiratory pressure
- Cdyn, dynamic lung compliance
- CPAP, continuous positive airway pressure
- EELV, end expiratory lung volume
- EMGdi
- diaphragmatic electromyography
- EMGic, intercostal electromyography
- PEEP, positive end expiratory pressure
- PEEPi, intrinsic PEEP
- PEEPe, extrinsic PEEP
- PTPdi, diaphragmatic pressure time product
- PTPoes, oesophageal pressure time product
- Poes, oesophageal pressure. Pga, gastric pressure
- Pao, airway opening pressure
- Pdi, transdiaphragmatic pressure
- Ptp, transpulmonary pressure
- Rrs, total lung resistance
- Ti, inspiratory time
- Te, expiratory time
- Ttot
- respiratory cycle time
- V̇i, minute ventilation
- Vt, tidal volume
- Vt/Ti, mean inspiratory flow
- Vt
- Te, mean expiratory flow
Statistics from Altmetric.com
- chronic obstructive pulmonary disease
- continuous positive airway pressure
- positive end expiratory pressure
- Cdyn, dynamic lung compliance
- CPAP, continuous positive airway pressure
- EELV, end expiratory lung volume
- EMGdi
- diaphragmatic electromyography
- EMGic, intercostal electromyography
- PEEP, positive end expiratory pressure
- PEEPi, intrinsic PEEP
- PEEPe, extrinsic PEEP
- PTPdi, diaphragmatic pressure time product
- PTPoes, oesophageal pressure time product
- Poes, oesophageal pressure. Pga, gastric pressure
- Pao, airway opening pressure
- Pdi, transdiaphragmatic pressure
- Ptp, transpulmonary pressure
- Rrs, total lung resistance
- Ti, inspiratory time
- Te, expiratory time
- Ttot
- respiratory cycle time
- V̇i, minute ventilation
- Vt, tidal volume
- Vt/Ti, mean inspiratory flow
- Vt
- Te, mean expiratory flow
In normal subjects the end expiratory lung volume (EELV) corresponds to the relaxation volume (Vr) or elastic equilibrium volume of the respiratory system. In patients with chronic obstructive pulmonary disease (COPD), because of severe airflow obstruction, the time available for expiration may be insufficient to allow the system to return to Vr. The residual inward elastic recoil creates a positive alveolar pressure at end expiration, known as intrinsic positive end expiratory pressure (intrinsic PEEP or PEEPi).1 In order to initiate inspiratory airflow, the respiratory muscles must generate a negative pressure equal in magnitude to PEEPi. Intrinsic PEEP has therefore been described as an inspiratory threshold load on the inspiratory muscles, increasing work of breathing. The presence of PEEPi also implies dynamic hyperinflation, with consequent worsening of thoracic wall geometry and muscle length-tension relationships. This further increases the workload of muscles as their efficiency and mechanical advantage are reduced.
Intrinsic PEEP has commonly been identified in patients with COPD during acute exacerbations, either during invasive or non-invasive ventilation.2–6 According to the “waterfall theory”, if PEEPi is the result of expiratory flow limitation, then application of extrinsic PEEP (PEEPe) at the airway opening should decrease the pressure gradient between the mouth and alveoli at end expiration (the inspiratory threshold load).7,8 This should be achieved without further hyperinflation. A number of studies in patients during acute exacerbations of COPD have demonstrated this effect, although complete counterbalancing of PEEPi has never been achieved and minor degrees of hyperinflation were produced.2–6 In mechanically ventilated patients a PEEPe of 85% of “static” PEEPi has been recommended to achieve maximal reduction of PEEPi with minimal increase in end expiratory lung volume.5
Chronic nocturnal non-invasive ventilation (NIV) via facemask has been proposed as a therapeutic modality in hypercapnic COPD. However, in contrast to patients with neuromuscular disease in whom this is now an established treatment option, trials in COPD have met with mixed success.9–11 One reason may be the existence of unrecognised PEEPi in these subjects12 which prevents optimal reduction in work of breathing and impairs patient-ventilator interaction.13 If the results in ventilated patients during acute exacerbations could be replicated in stable COPD, it is possible that the addition of targeted PEEPe could improve the outcomes of NIV in patients with severe stable COPD. Petrof et al14 achieved reductions in indices of respiratory muscle effort by application of CPAP during sleep in severe stable COPD. They suggested that this resulted from reductions in PEEPi, but PEEPi was not actually measured. We therefore sought to document the effect of increasing PEEPe on inspiratory threshold load, lung volume, and work of breathing in a group of subjects with severe stable COPD.
METHODS
Subjects
Nine men with severe stable COPD were studied. The diagnosis was made clinically and supported by lung function data. No subject had experienced an exacerbation of his airways disease during the previous 2 months. Their clinical characteristics and lung function data are shown in table 1. Four were being treated with long term nocturnal bilevel ventilation. The subjects were studied in the morning following a dose of their usual bronchodilator. They were seated on a bed with the backrest inclined at 45 degrees. All subjects gave informed written consent and the study protocol was approved by the research and ethics committee of the Repatriation General Hospital, Daw Park.
Patient characteristics
Protocol
Oesophageal and gastric pressures were measured by conventional balloon catheter systems inserted via the nose after topical anaesthesia. Oesophageal (Poes), gastric (Pga), and airway opening (Pao) pressures were recorded using pressure transducers (Spectramed DTX, Oxnard, USA) calibrated against a water manometer. Subjects breathed via a sealed facemask (n=4) (Hans Rudolph, Kansas City, MO, USA) or a mouthpiece (n=5), depending on comfort. When using a mask, a narrow perforated tube connected to a capnometer (POET II model 602-3 Criticare Systems, Waukesha, WI, USA) was positioned encircling the mask to detect leaks. A heated pneumotachograph (PT36, Erich Jaeger, Germany) attached to a differential pressure transducer (Erich Jaeger, Germany) was positioned between the mask and the bias flow valve of the CPAP pump to measure inspiratory and expiratory flow (V̇). Volume (V) was obtained by electrical integration of flow. Surface diaphragmatic (EMGdi) and parasternal intercostal (EMGic) EMG activity were recorded. The signals were bandpass filtered between 30 and 1000 Hz and notch filtered at 50 Hz. Rib cage (RC) and abdominal (AB) excursions were measured by respiratory inductive plethysmography (RIP) (Ambulatory Monitoring Inc, Ardsley, NY, USA) calibrated over a 10 minute period using the qualitative diagnostic calibration described by Sackner et al.15 The electrocardiogram was also monitored (Hewlett-Packard 78342, Andover, USA). Continuous positive airway pressure (CPAP) was applied using a commercial pump (Sullivan model APD2E, ResMed, Sydney, Australia) modified to decrease the minimum CPAP, with a plateau exhalation valve (Respironics, Murraysville, PA, USA) to minimise rebreathing.
Once the subject was in a stable breathing pattern and following RIP calibration, he was asked to stop breathing briefly in order to check the pneumotachograph zero flow baseline. Likewise, the Poes, Pga, and Pao transducers were opened to the atmosphere to check each pressure baseline. On resumption of stable breathing the next 15–30 breaths were used for analysis. CPAP was then applied and increased in increments of 1 cm H2O to a maximum of 10 cm H2O. After each increase, and following a 3 minute stabilisation period, flow and pressure baselines were again rechecked and then data from 15–30 breaths were acquired for detailed analysis.
Data analysis
All signals were recorded on an IBM laptop computer using a commercial data acquisition system with a sampling rate of 1000 Hz per channel (Dataq Instruments Inc, OH, USA). Thereafter, data were analysed using custom written software. To allow for small between subject differences in applied CPAP, each variable was subsequently determined in every subject at precise intervals of 1 cm H2O CPAP using linear interpolation.
Dynamic intrinsic PEEP was calculated as the negative deflection in Poes preceding the start of inspiratory flow (fig 1). If PEEPi is the result of expiratory muscle activity during expiration, the fall in oesophageal pressure prior to initiation of flow does not represent an inspiratory threshold load on inspiratory muscles, but merely the relaxation of expiratory muscles. We therefore measured the deflection in transdiaphragmatic pressure (Pdi) over the same interval as Poes. When the fall in Poes is caused by relaxation of expiratory muscles, Pga should fall simultaneously with Poes, and the magnitude of the change in Pdi should be substantially less than that of Poes.6 For the same reason we monitored thoracic and abdominal wall motion using RIP. If there is relaxation of abdominal expiratory muscles at end expiration, abdominal dimensions should simultaneously increase.16
Experimental record of four breaths from subject 8. The oesophageal pressure trace (Poes) demonstrates recurrent pressure fluctuations centred on the R wave of the ECG due to cardiac pulsation, making it difficult to discern the true Poes fluctuations. By ensemble averaging of >100 beats the mean cardiac artefact was identified. This was subtracted from the raw Poes to give a “filtered” Poes trace. Differentiation of this signal gives the instantaneous change in slope. The onset of effort was then identified as the last time dPoes/dt passes through 0 at initiation of inspiratory effort. The first vertical line indicates this point, while the second corresponds to onset of flow. PEEPi was calculated as the change in filtered Poes between these two lines. Transdiaphragmatic pressure (Pdi) was similarly filtered and the change over the same time interval recorded. EMG signals were full wave rectified and moving time averaged with a time constant of 100 ms (MTA EMGdi).
The Pga, Poes, Pdi and flow traces of all breaths were individually examined. Sighs, swallows, and breaths with a tidal volume (Vt) below 300 ml were excluded from further analysis as were all breaths during which leaks occurred. We found that, despite optimal balloon placement, there was still substantial cardiac artefact on Poes and Pga tracings. This made it difficult to identify reliably the exact point of inflection in Poes (start of inspiratory effort). Therefore, at each CPAP pressure the mean cardiac component of the Poes and Pga records (artefact) was calculated over a period of 2–3 minutes by ensemble averaging the recurrent pressure fluctuations centred on the R wave of the ECG. The cardiac artefact was found not to vary substantially within the tidal volume range. The mean artefact was then subtracted from the Poes and Pga traces at each cardiac cycle. Figure 1 shows the results of this filtering process in one patient. The filtered Poes signal was differentiated to give the instantaneous slope of pressure v time. This exaggerates the point of inflection of the trace (fig 1). The pressure-time product of the respiratory muscles (PTPoes/min) and of the diaphragm (PTPdi/min) were calculated17 and expressed per minute. Tidal variations in Poes (ΔPoes) and Pdi (ΔPdi) were calculated relative to initiation of effort. Total lung resistance (Rrs) was calculated at an inspiratory flow of 200 ml/s using the Mead-Whittenberger method.18
ECG artefact was removed from EMG signals in the same manner as for Poes and Pga. Filtered EMG signals were full wave rectified and moving time averaged with a time constant of 100 ms. Peak phasic EMG was expressed as an absolute value in arbitrary units and as a percentage of tonic activity (minimum activity in the last 400 ms of the preceding expiration).
Mean respiratory cycle time (Ttot), tidal volume (Vt), inspiratory and expiratory time (Ti and Te), inspiratory and expiratory flow (Vt/Ti and Vt/Te), and inspired minute ventilation (V̇i) were calculated from the flow record. Changes in end expiratory lung volume (ΔEELV) with increasing CPAP were calculated by plotting end expiratory transpulmonary pressure (Ptp) on the dynamic lung compliance curve.6 The slope of the pressure-volume relationship during tidal breathing did not change as CPAP increased.
Statistics
The effect of increasing CPAP on each parameter was examined by repeated measures ANOVA using the Greenhouse-Geisser correction for multisample asphericity. Appropriate reductions in degrees of freedom were used to account for missing values as not all subjects tolerated the highest CPAP pressures. Two way ANOVA for repeated measures was used to test whether PEEPi, as measured by Poes and by Pdi, were significantly different as CPAP increased. A p value of <0.05 was accepted as statistically significant. All results are expressed as mean (SE).
RESULTS
Intrinsic PEEP was detected in all patients. However, in patient 4 significant expiratory muscle activity was detected at baseline, and data from this patient were excluded from further analysis. Intrinsic PEEP in the remaining eight patients was 2.86 (0.60) cm H2O. CPAP reduced PEEPi in a linear fashion in all subjects reaching a minimum of 0.96 (0.16) cm H2O at the highest CPAP (p<0.05, fig 2). At an interpolated CPAP pressure of 2.86 cm H2O (equal to the initial PEEPi level), PEEPi was still 2.4 (0.7) cm H2O. Two other patients developed expiratory muscle activity at higher CPAP pressures, resulting in a gradual divergence of PEEPi as measured by Poes and by Pdi (CPAP by method of measurement interaction, p<0.05; fig 2).
Effect of CPAP on PEEPi as measured by Poes (solid line) and the simultaneous change in Pdi (dashed line) in the eight subjects who did not initially display expiratory muscle activity. The gradual divergence of the traces is due to onset of expiratory muscle activity in two other subjects (p<0.05, two way ANOVA for measurement method by CPAP interaction; p<0.05 by both methods, repeated measures ANOVA).
There was no significant change in Ti, Te, Ttot, Vt, Vt/Ti, or Vt/Te with increasing CPAP. Small but consistent changes in Vt led to a significant increase in V̇i at higher pressures (from 9.2 (0.6) l/min to 10.7 (1.1) l/min, p<0.01; fig 3). There was a trend to a reduction in Rrs which fell from 15.5 (5.0) cm H2O/l.s at 0 cm H2O to 3.5 (0.6) cm H2O/l.s at 10 cm H2O. However, this failed to reach statistical significance (p=0.08, fig 4).
Effect of CPAP on minute ventilation (Vi; p<0.05).
Effect of CPAP on inspiratory resistance (Rrs) at inspiratory flow of 200 ml/s (NS).
EMGdi did not change with CPAP, but EMGic increased both in absolute terms (from 0.37 (0.07) to 0.44 (0.11) arbitrary units) and as a percentage of tonic values (from 353 (37)% to 467 (100)%). In the latter case this reached statistical significance (p<0.05, fig 5).
Influence of CPAP on (A) diaphragmatic (EMGdi) and (B) intercostal (EMGic) electromyographic activity. Peak phasic EMG activity is expressed as a percentage of tonic activity. p<0.05 for EMGic.
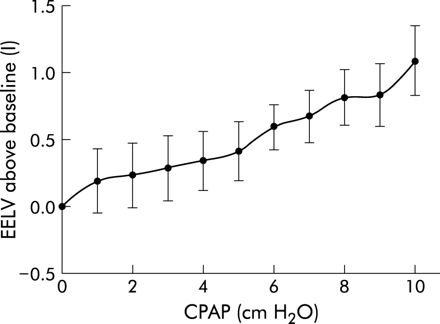
PTPdi/min and ΔPdi were reduced by a maximum of 53.4 (9.0)% and 47.7 (6.1)%, respectively (p<0.05, fig 6). Lesser non-significant reductions were achieved in PTPoes/min and ΔPoes (32.3 (7.5)% and 33.4 (9.4)%). These reductions in indices of muscle effort were achieved at the expense of an increase in EELV of 1.10 (0.26) l (p<0.01, fig 7).
Effect of CPAP on indices of diaphragmatic effort. (A) Pressure-time product of diaphragm per minute (PTPdi/min; p<0.05). (B) Tidal swings in transdiaphragmatic pressure (ΔPdi; p<0.05).
Increase in end expiratory lung volume (EELV) with CPAP (p<0.01).
DISCUSSION
The major findings of this study were that, in stable patients with COPD who have measurable PEEPi, the application of CPAP has minimal effects on the inspiratory threshold load until CPAP levels greatly exceed PEEPi. At these pressures, while there is a reduction in indices of respiratory muscle effort, this is at the expense of substantial increases in lung volume as well as some expiratory muscle recruitment.
In contrast, a number of studies in patients during acute exacerbations of COPD have demonstrated the ability of PEEPe to diminish PEEPi, the inspiratory threshold load, and work of breathing without a significant increase in lung volume.2–6 The “waterfall theory”7,8 predicts that PEEPe should only diminish PEEPi without worsening hyperinflation if PEEPi is due to expiratory flow limitation. Flow limitation is most definitively assessed by applying negative pressure to the airway opening during expiration.19 We did not perform this test and therefore cannot be certain what contribution, if any, flow limitation made to PEEPi. Other possible mechanisms causing PEEPi include (1) expiratory muscle activity; (2) post-inspiratory activity of inspiratory muscles; (3) glottic narrowing during expiration; and (4) intrinsic airway narrowing.
Expiratory muscle activity is known to contribute to PEEPi, although it does not contribute to the inspiratory threshold load.16 While we believe the methods used to detect expiratory activity in the abdominal muscles were reliable, a contribution from chest wall muscles independent of abdominal muscles cannot be excluded. We did detect expiratory muscle activity in one patient at baseline, and expiratory activity became evident in two others at high levels of CPAP (fig 2).
Post-inspiratory activity of inspiratory muscles is known to contribute to dynamic hyperinflation in asthma.20 In COPD, inspiratory muscle activity does not appear to persist far into expiration,21 and would therefore be unlikely to contribute significantly to PEEPi.
Asthmatic patients also brake expiration through constriction of the glottis and supraglottic area during induced bronchoconstriction, and this constriction is abolished by CPAP.22 We are unaware of any investigation of expiratory glottic narrowing in COPD. Lastly, it is possible that intrinsic airway narrowing without dynamic airway compression may have retarded airflow, contributing to PEEPi.
However, we believe it is likely that our patients were flow limited; certainly, flow limitation is common in severe stable COPD.19 As illustrated by the example in fig 1, the expiratory flow time profile of all our patients was typical of flow limitation. It is notable that no study, either in mechanically ventilated or spontaneously breathing patients, has shown that PEEPi can be reduced to zero, even by pressures considerably in excess of PEEPi.2–6 Gay et al23 and Georgopoulos et al,4 using isovolume pressure-flow loops, showed that flow limitation is not an “all or none” phenomenon. In their studies there was an inconsistent relationship between baseline PEEPi and the critical PEEPe level which begins to retard expiratory flow and increase lung volume. It can be envisaged that, in a non-homogeneous lung, there is a wide variation in time constants between lung units. Low levels of PEEPe may counterbalance the PEEPi of faster units (with lower PEEPi) and then begin to hyperinflate them while PEEPi remains in units with long time constants. Flow limited and non-flow limited pathways may coexist in the same subject. The consequences of application of CPAP in terms of PEEPi and EELV in any patient will therefore depend on the proportion of PEEPi due to flow limitation and on the distribution of PEEPi levels between different lung units. It follows that the response of an individual patient to CPAP is not predictable by their initial PEEPi. In fact, some studies have shown increased PEEPi with low levels of CPAP,24 suggesting ventilation of previously functionally closed lung units with presumably high levels of PEEPi.
Only one previous study has evaluated the effect of extrinsic PEEP on PEEPi in patients with stable COPD.25 Seven subjects had a mean PEEPe of 3.2 cm H2O added to inspiratory pressure support (PS) of 8.1 cm H2O and 15.9 cm H2O. Dynamic PEEPi was reduced from 2.5 (1.3) cm H2O to 1.05 (0.7) cm H2O with the lower PS level and from 2.6 (1.0) cm H2O to 0.9 (0.5) cm H2O at the higher level. PTPoes and PTPdi were reduced. EELV (measured by RIP) increased by 327 (118) ml compared with control values at PS 15.9/PEEP 3.2, although PS 15.9 alone increased EELV by 100 ml. These results differ substantially from ours where significant reductions in PEEPi were only achieved by CPAP levels greatly in excess of initial PEEPi, and then only with substantial increases in EELV. The patients in the study by Nava et al had more severe lung function impairment than ours (mean FEV1 20% predicted) which may have produced a greater degree of flow limitation. The authors also conceded that measurements of dynamic PEEPi may have been affected by patient/circuit compliance and ventilator trigger function.25
Given the modest reductions in inspiratory threshold load, it is surprising we achieved such large reductions in indices of inspiratory work. This may have been due to concomitant reductions in upper or lower airways resistance. We calculated total inspiratory resistance (Rrs) at V̇=200 ml/s using the Mead-Whittenberger technique.18 While there was a strong trend towards lower Rrs with increasing CPAP, this failed to reach statistical significance (p=0.08, fig 4). It is also possible that, with reduced pleural pressure swings and increased absolute pleural pressures, chest wall deformation during inspiration was reduced. Chest wall distortion is significant in severe COPD and has been shown to increase work of breathing.26 A further possibility is that cyclical airway closure and reopening was prevented by increased lung volume.27 However, under these circumstances lung compliance would be expected to increase with increasing CPAP. Dynamic compliance, at least, did not change during the application of CPAP in our study.
PTPoes and PTPdi have been shown to correlate well with diaphragmatic oxygen consumption.17 However, these experiments were performed at constant lung volume. The Hill equation28 indicates that the energy cost of skeletal muscle contraction depends on the tension developed, the duration of contraction, and the velocity of shortening. As lung volume increases, the diaphragmatic tension required to produce a given Pdi or Poes increases due to worsening mechanical advantage and length-tension relationships. Therefore, while reductions in PTP values in our subjects with increasing CPAP may give the impression of reduced muscle work, diaphragmatic oxygen consumption may, in fact, have remained stable or even risen. Collett et al29 showed an increase of 41% in oxygen cost of breathing at constant load, ventilation, work rate, and PTPoes in normal subjects when EELV was increased from functional residual capacity by 37% of inspiratory capacity.
Changes in lung volume may also affect EMGdi signals. With increasing lung volume and diaphragmatic descent, the electrode site of maximum EMGdi amplitude moves caudally.30 It is therefore possible that stable EMGdi peak amplitudes in our data reflect increasing diaphragmatic electrical activity. EMGic, which is less susceptible to artefact with changing lung volume, increased with increasing CPAP. Alternatively, stable EMGdi with increasing EMGic together with greater reductions in PTPdi than PTPoes may indicate a transfer of workload from diaphragm to intercostal muscles at higher lung volumes.
Methodological issues
Detection of expiratory muscle activity
Expiratory muscle activity is known to contribute to PEEPi.16 We compared the fall in Poes and the simultaneously measured rise in Pdi in an attempt to detect a contribution of expiratory muscles to measured PEEPi. At the same time we monitored thoracoabdominal movements using RIP. Previous work has described a precipitous fall in Pga at end expiration in combination with outward movement of the abdominal wall in all patients in whom expiratory muscle activity was detected.16,31 Attempts have been made to calculate a “corrected” PEEPi but their accuracy has been questioned.31 We therefore did not attempt such a correction. It is possible that there was expiratory activity of the chest wall muscles alone that was not detected by this method. While this cannot be excluded, it appears unlikely that chest wall expiratory muscles would be active without simultaneous activity of abdominal muscles.
Measurement of change in EELV (ΔEELV)
It was initially our intention to use RIP in DC mode to measure ΔEELV. However, in preliminary experiments we found that, despite allowing 1 hour “warm up time” at body temperature and taping the bands in place, the RIP signal was subject to both gradual baseline drifts and also to more rapid (over 3–4 breaths) shifts. We and others32 also found that, under positive pressure breathing, RIP calibration deteriorated when compared with simultaneously measured Vt using the pneumotachograph. Validation experiments have shown RIP to have only moderate accuracy in measuring changes in EELV, even over short periods of 1–3 minutes.15,32 We therefore felt that RIP was inadequate to monitor ΔEELV over 60–90 minutes, as required in our experiment.
We chose instead to calculate ΔEELV by plotting end expiratory transpulmonary pressure on the dynamic lung compliance (Cdyn) curve. Other studies have documented minimal change in compliance with application of CPAP in patients with COPD,2–6 and the change in EELV has previously been assessed in this manner.6 While we believe this method provides an approximate estimate of ΔEELV, we cannot be certain about its precision. However, we believe the progressive change in end expiratory transpulmonary pressure observed in all our subjects strongly suggests that lung volume increased, even with very low levels of CPAP.
In conclusion, we have found that application of CPAP in stable patients with severe COPD has minimal effects on the inspiratory threshold load until CPAP levels greatly exceed PEEPi. At these pressures we observed substantial increases in lung volume as well as some expiratory muscle recruitment. Indices of respiratory muscle effort decreased, but these decreases may not have been truly indicative of reduced muscle energy consumption. Thus, based on current evidence, application of CPAP in severe stable COPD should be approached with caution. Low levels of extrinsic PEEP (up to 5 cm H2O) as are commonly used in NIV do not counterbalance intrinsic PEEP. Higher levels of CPAP do reduce the inspiratory threshold load which may improve patient ventilator synchrony. However, the resultant gains may be counterbalanced by increases in muscle effort due to increased lung volumes.
Acknowledgments
The authors gratefully acknowledge the assistance of Robin Woolford, Department of Biomedical Engineering, Repatriation General Hospital, Daw Park for technical assistance in this study. Supported by grants from the National Health and Medical Research Council of Australia and Air Liquide Australia.
REFERENCES
Footnotes
-
This study was supported by the National Health and Medical Research Council of Australia and the Daw Park Research Foundation.