Article Text
Abstract
BACKGROUND Many patients continue to take regular β agonists, often at high doses, contrary to national and international guidelines. Some studies have suggested that this can worsen asthma control, but whether such patients can reduce their dose of β agonist and whether they would benefit from this has not been determined. Reduction of β agonist dose was studied in a placebo controlled parallel group study.
METHODS Following a run in period, 33 subjects with asthma taking regular β agonists were converted to an equivalent dose of terbutaline via a Turbohaler. Two weeks later terbutaline was continued at the same dose or changed to placebo in two stages a week apart. The change over period was covered by an increased dose of inhaled steroid to attenuate any immediate effects of the change in dose. Subjects then attended weekly for six weeks for measurement of forced expiratory volume in one second (FEV1) and the dose of methacholine that produced a 20% fall in FEV1 (PD20). Peak expiratory flow (PEF) and symptom scores were recorded twice daily throughout the study. Exacerbations, lung function, bronchial responsiveness, bronchodilator response, β agonist use, and symptoms were compared before and six weeks after reduction in the dose of β agonist.
RESULTS Twenty five of the 33 subjects completed the study; three patients in each group withdrew due to an asthma exacerbation. The median terbutaline dose fell from 2500 to 500 μg/day in the β agonist reduction group and from 3000 to 2250 μg/day in the control group. There were small non-significant changes in FEV1, PEF, symptom scores and PD20 methacholine over the course of the study. The FEV1 response to a β agonist was greater in those who reduced their β agonist dose than in the control group although the final FEV1 achieved was the same.
CONCLUSIONS Patients with asthma taking high doses of β agonists can reduce the amount of β agonist they use without a significant change in their asthma control. There was no evidence of improved asthma control with β agonist dose reduction.
- β agonist
- dose reduction
- asthma
Statistics from Altmetric.com
The use of high dose preparations of non-selective or less β2 selective β agonists (isoprenaline and fenoterol) has been associated with epidemics of asthma deaths1-3although a causal relationship has been disputed. Regular exposure to β agonists has had deleterious effects on lung function and bronchial responsiveness in some short term studies once their acute bronchodilator effect had worn off.4-6 Longer term clinical studies have shown little or no benefit from regular short acting β agonists compared with placebo7-10 and, in one instance, poorer asthma control was reported.11 Against this background national and international guidelines recommend that β agonists should, when possible, be used on an “as needed” basis. Nevertheless, many subjects with asthma continue to inhale β agonists regularly, often in high doses.
If regular use of β agonists causes asthma to deteriorate, patients taking regular β agonists at high doses might be expected to improve with dose reduction. In two uncontrolled studies in the 1960s most patients taking very high doses of isoprenaline showed clinical improvement and a marked increase in forced expiratory volume in one second (FEV1) when they reduced their use of isoprenaline.12 ,13 The effect of a reduction in dose of β agonist has not been studied in a controlled trial, however. We have examined the effects of β agonist dose reduction in a placebo controlled trial in 33 subjects taking moderately high doses of β agonists. Since abrupt reduction in β agonist use in subjects who are tolerant to their effects could lead to a temporary deterioration in asthma control, the β agonist reduction period was covered by an increased dose of inhaled steroid for 18 days. FEV1, peak expiratory flow (PEF), bronchial responsiveness to methacholine, symptom scores, and β agonist response were compared in the two groups before and six weeks after dose reduction (five weeks after cessation of the extra inhaled steroid).
Methods
SUBJECTS
To enter the study subjects had to be aged 17–65 years, be taking an inhaled β agonist at a dose equivalent to at least 200 μg salbutamol four times daily and inhaled beclomethasone dipropionate or budesonide up to 1200 μg daily, and to have an FEV1between 50% and 90% predicted. No other asthma treatments were allowed. Asthma had to be stable on entry with no exacerbation or change in treatment in the six weeks prior to the study. Subjects gave written informed consent to the study which was approved by the Nottingham City Hospital ethics committee.
MEASUREMENTS
FEV1 was measured with a dry bellows spirometer (Vitalograph, Vitalograph Ltd, Bucks, UK) as the higher of two measurements within 100 ml and PEF as the best of three readings using a mini-Wright peak flow meter (Airmed UK). Bronchial responsiveness was measured with a modification of the method of Yanet al.14 Subjects inhaled three puffs of saline from a DeVilbiss nebuliser whilst breathing in slowly from functional residual capacity to total lung capacity, followed by doubling doses of methacholine from 0.048 to 49 μmol. FEV1 was measured one minute after each dose and the test was stopped when FEV1 had fallen by 20% from the post saline value. The provocative dose of methacholine required to cause a 20% fall in FEV1 (PD20) was calculated by linear interpolation of the last two readings on the log dose-response plot. Bronchodilator dose-response was measured as change in FEV1 after cumulative doses of 250, 500 and 750 μg terbutaline given at 15 minute intervals by Turbohaler. Subjects were instructed to withhold bronchodilator medication for four hours prior to the study visits which were at the same time of day. Subjects kept diary cards throughout the study recording twice daily PEF, all medication used, and symptom scores (from 0 = no symptoms to 4 = severe symptoms).
PROTOCOL
This was a double blind placebo controlled study in which subjects were randomised to reduce their dose of β agonist (reduction group) or not (control group). Subjects entered a two week run in period during which they recorded their β agonist use. Their usual β agonist was then replaced with an equivalent dose of terbutaline administered via two inhalers (Turbohaler), each giving half the dose (250 μg per puff) and labelled A and B. It was assumed that 500 μg terbutaline was equivalent to 200 μg salbutamol. Subjects were also given a standard terbutaline Turbohaler (500 μg per puff) to be used as relief medication throughout the study. After a further two weeks subjects attended the clinic for measurement of FEV1 and PD20 methacholine (baseline values) and budesonide 400 μg twice daily by Turbohaler (inhaler C) was added to their medication for 18 days. Seven days after starting inhaler C they were randomised, using a computer random number table, to reduce their β agonist dose or not. This was achieved by replacing inhaler A with placebo or the same dose of terbutaline and one week later by replacing inhaler B in the same way. Subjects were seen weekly for a further six weeks for measurement of FEV1 and PD20 methacholine. A bronchodilator dose-response study was carried out during week 4 and at the end of the study in week 12. Subjects were withdrawn if their FEV1 fell by 15% from baseline, if they had a 20% fall in PEF on three consecutive days, or if there was any change in their asthma medication.
The primary end points were change in FEV1 and PEF from baseline to the end of the study six weeks after β agonist dose reduction and five weeks after stopping the extra inhaled steroid. Secondary end points included change in methacholine responsiveness, symptom scores, and β agonist response. Baseline measurements were made during and at the end of week 4 when subjects had used the study terbutaline inhalers for two weeks without dose reduction and had not started the extra budesonide. Thirty subjects provided 80% power to detect a difference of 300 ml in FEV1 between treatment groups at a significance level of 0.05 according to a previous study.15
ANALYSIS OF DATA
Baseline values for mean PEF and median symptom scores and β agonist use were calculated from recordings at week 4. Reversibility to terbutaline was characterised by initial and maximum FEV1and the increase in FEV1 following terbutaline. PD20 methacholine values were log transformed for analysis and differences in PD20 were expressed in doubling doses. Treatment effect was expressed as change from baseline (week 4) to an average value from weeks 11 and 12 and compared between groups by the Student’s t test for independent samples on parametric data and by the Mann-Whitney test for β agonist dose and symptom scores. Mean and median values with 95% confidence intervals (95% CI) and interquartile ranges (IQR) are given where appropriate.
Results
Thirty three subjects (15 women) took part in the study, 15 in the β agonist reduction group and 18 in the control group. Eight subjects withdrew from the study, one from each group for personal reasons and three from each group due to an exacerbation of their asthma, leaving 25 who completed all 11 visits. Baseline characteristics of the reduction and control groups were similar (table 1). Prior to randomisation patients were taking a median dose of 3000 (range 2000–7000) μg terbutaline a day with no significant difference between the two groups (table 1).
FEV1, PEF, PD20 methacholine, symptom scores, and terbutaline dose at baseline and end of study in the β agonist reduction group and controls
β AGONIST DOSE
The total amount of β agonist inhaled daily (regular plus relief) remained reasonably constant in the control group, being 3000 μg at week 4 and 2250 μg at week 12, whereas in the dose reduction group it fell significantly from 2500 to 500 μg daily (fig1). The difference in β agonist dose reduction from weeks 4 to 12 between groups was 1500 μg terbutaline per day (95% CI 750 to 1750; p<0.002).
Median dose (μg/day) of relief terbutaline (top) and total terbutaline (bottom) inhaled by the reduction (●) and control (*) groups. (BUD = 18 day period of budesonide 800 μg/day).
The dose of relief terbutaline increased slightly in the β agonist reduction group during weeks 5 and 6 as the study β agonist was withdrawn, but then declined again and by week 8 there was no difference between the groups (fig1).
LUNG FUNCTION, BRONCHIAL REACTIVITY, AND SYMPTOMS
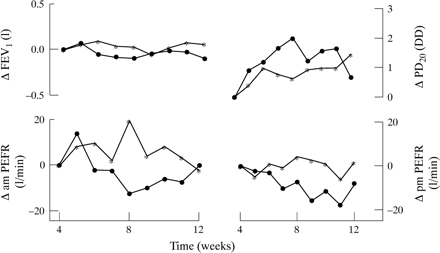
There were small changes in FEV1, morning and evening PEF, symptom scores, and PD20 methacholine between week 4 and the end of the study but none of the differences was statistically significant (fig 2). The mean difference (95% CI) for change in FEV1 was 0.1 (–0.5 to 0.7) litres, for morning and evening PEF was 4 and 9 (–76 to 84 and –67 to 85) l/min, and for PD20 was 0.3 (–1.06 to 1.73) doubling doses.
Change in forced expiratory volume in one second (FEV1), morning and evening peak expiratory flow rate (PEFR), and dose of methacholine causing a 20% fall in FEV1 (PD20 methacholine) in the β agonist reduction group (●) and control group (*).
On the bronchodilator response day the increase in FEV1 in response to terbutaline rose from 8.4% at week 4 to 14.9% at week 12 in the β agonist reduction group and fell from 17.7% to 9.7% at the same times in the control group (table 2). The change in the response to terbutaline in the two groups differed by 14.5% (95% CI 1.1 to 27.9, p = 0.03) although there was no significant difference in maximum FEV1 achieved between the two groups at week 12 (difference 0.26 (95% CI –2.9 to 0.8, p = 0.3) litres).
Mean (SE) forced expiratory volume in one second (FEV1) before and after a cumulative dose of 1500 μg terbutaline by Turbohaler and percentage change in the β agonist reduction and control groups
Discussion
This is the first attempt to determine, under placebo controlled conditions, whether patients using moderately high doses of β agonists are able to reduce their β agonist use and, if so, what affect this has on lung function, bronchial responsiveness, and symptom scores. The patients in this study were able to reduce their β agonist use considerably but without any evidence of improved asthma control.
Before discussing the implications of our findings some methodological points require comment. Firstly, because we excluded patients with unstable asthma and those using high doses of inhaled steroids or other asthma medication, recruitment was difficult. In addition, six patients dropped out due to an asthma exacerbation. All the differences in clinical end points were small, however, and some way from being statistically significant so it is unlikely that a clinically important outcome has been missed. Secondly, we have no independent measure of treatment compliance or the accuracy of diary recordings although the importance of both was emphasised strongly at each visit.
Thirdly, during the run in period patients were changed from their usual β agonist to terbutaline by Turbohaler to allow us to change their treatment under double blind conditions later. Most patients had been taking salbutamol by metered dose inhaler and, from data available at the time, we assumed 200 μg salbutamol by metered dose inhaler to be broadly equivalent to 500 μg terbutaline by Turbohaler. Work published subsequently suggests that lung deposition is likely to be greater when drugs are administered by Turbohaler compared with a metered dose inhaler,16 ,17though the findings have varied18 and this is less likely to be true for patients who had been using a spacing device with their metered dose inhaler.19 The dose of β agonist probably increased to some extent therefore in most subjects at week 2 when they were converted to terbutaline via the Turbohaler. Despite this, both groups used more terbutaline (regular plus relief) between weeks 2 and 4. This may be because subjects were allowed to increase but not decrease their dose during this time or it may be that some β agonist was taken by habit or for non-asthma symptoms. Assuming the Turbohaler delivers approximately twice as much drug to the airways, the final dose of β agonist was approximately equivalent to 400 μg salbutamol in the reduction group and 1800 μg in the control group. Dose reduction was still achieved in the reduction group therefore, but the control group actually increased their β agonist dose compared with baseline.
Finally, we included 18 day steroid cover to try to reduce any transient withdrawal effects following β agonist dose reduction. This should not have affected the outcome of the study since both groups were treated in the same way and because the main end points were measured five weeks after stopping the extra inhaled steroid.
The changes in lung function and bronchial reactivity between baseline and the end of the study were small and did not differ significantly between the two groups. Although subjects were asked not to take terbutaline within four hours of study visits or peak flow recordings, measures of lung function may have been more affected by terbutaline treatment in the control group since the doses they were taking were considerably higher than the reduction group. This could have masked a small benefit from β agonist dose reduction on lung function and bronchial responsiveness.
The only significant difference to emerge between the two groups was the change in response to terbutaline between baseline and week 12. The increase in reversibility in the β agonist reduction group was largely due to the lower pre-terbutaline FEV1 on the reversibility day in week 12 compared with week 4. The reduction in reversibility in the control group would be consistent with the downregulation of β receptors seen in vitro,20 which could have resulted from the increase in β agonist dose when the subjects changed from a metered dose inhaler to a Turbohaler. It may also be due to chance since most studies in subjects with asthma have not shown significant changes in bronchodilator responsiveness following an increased dose of a short acting β agonist.21
Despite anecdotal reports of improved asthma control with β agonist dose reduction, only two previous uncontrolled studies have addressed this. Both were carried out nearly 30 years ago and involved excessive use of isoprenaline from inhalers or hand held nebulisers. Symptoms improved with cessation of isoprenaline in 30 patients with severe refractory asthma12 and in seven of eight symptomatic patients taking high doses of isoprenaline, some of whom had a bronchoconstrictor response to acute inhalation of isoprenaline.13 There are considerable differences between our study and the two earlier studies, however. Isoprenaline is a non-selective β agonist and patients were taking considerably higher equivalent doses than the doses of terbutaline taken in our study. Our subjects had better controlled asthma, were taking regular inhaled steroids, and none had a fall in FEV1 following terbutaline inhalation.
In conclusion, patients taking moderately high doses of inhaled β agonists were able to reduce their dose considerably with no deterioration in asthma control. We found no evidence of improved asthma control although we excluded patients with the most severe and unstable asthma who might be those most likely to benefit from β agonist dose reduction. Patients on lower doses of β agonists had a greater acute response to an inhaled β agonist and this may be an advantage of taking lower doses. Our study supports the recommendations that β agonists should be used for symptomatic relief of asthma symptoms rather than on a regular basis.
Acknowledgments
The authors are grateful to The National Asthma Campaign for supporting the study, Dr Ian Pavord and Dr Jon Bennett for help with the study design, S Pacey (senior pharmacist) for storing and dispensing the treatments, and Astra Draco Ltd for supplying the terbutaline and placebo inhalers.