Article Text
Abstract
BACKGROUND Respiratory function in transplanted children is important because of the long life expectancy of bone marrow transplant recipients, particularly children. Attention is now being focused on the late sequelae of treatment on organ system function. A few papers have been published but available data are somewhat conflicting.
METHODS A cross sectional study aimed at evaluating the late effects of transplantation on lung function was performed in a group of 52 young patients who were given autologous or allogeneic bone marrow transplants during childhood for haematological malignancies.
RESULTS No patients reported chronic respiratory symptoms. The distribution of respiratory function patterns showed that only 62% of patients had respiratory function within the normal limits; 23% had a restrictive pattern and 15% had isolated transfer factor impairment. The percentage of patients with lung function abnormalities was higher in those who (1) received a bone marrow transplant after two or three complete remissions compared with those who were transplanted immediately after the first remission (54% vs 21%; p<0.02), (2) underwent allogeneic bone marrow transplantation rather than an autologous transplantation (45% vs 26%; p = 0.06), and (3) had a pulmonary infection compared with those without (56% vs 26%; p = 0.07).
CONCLUSIONS In spite of the absence of chronic respiratory symptoms there is a high prevalence of children with late pulmonary sequelae after bone marrow transplantation. Regular testing is recommended after transplantation, in particular in subjects at higher risk of lung injuries, such as those receiving transplants after more than one remission, those receiving allogeneic transplants, and those having suffered from pulmonary infections. When lung function abnormalities become apparent, long term follow up is necessary to see whether they become clinically relevant. All patients should remain non-smokers after transplantation and should have active early and aggressive treatment for respiratory illnesses.
- bone marrow transplantation
- lung function
Statistics from Altmetric.com
Recent advances in transplant immunobiology, supportive care, and prevention of graft versus host disease (GVHD) have increased the success of bone marrow transplantation (BMT). Considering the long life expectancy of bone marrow transplant recipients, particularly children, attention is now being focused on the late sequelae of treatment on organ system function.
Pulmonary complications are a major cause of morbidity and mortality in the early period after BMT.1 However, there is little published information on long term respiratory abnormalities in transplanted children and available data are somewhat conflicting.2-7 The variation between studies may result from the different underlying diseases, different types of transplantation, and the change in therapeutic strategies over time. Furthermore, there is uncertainty over the relationship between changes in lung function and the malignancy, the effects of toxicity of the drugs used to induce remission of the disease, those of the preparative regimens, type of transplant, and transplant related complications.6
We report here the results of a cross sectional study carried out on a group of young patients who were given autologous or allogeneic bone marrow transplants during childhood for haematological malignancies.
Methods
PATIENT SELECTION AND STUDY PROTOCOL
Between 1986 and 1994, 134 children with haematological malignancies underwent BMT in our Pediatric Department; 52 of the 71 survivors were included in this cross sectional study, 10 were living abroad and therefore could not be evaluated, and nine were not able to cooperate in performing lung function tests because they were under six years old. All patients were examined at least three years after transplantation (range 3–11 years). The characteristics of the population studied are reported in table 1.
Characteristics of patients included in the study
All information about the original malignant disease, first line treatment, conditioning regimen, type of BMT, donor, GVHD prophylaxis, and any early or late complications including respiratory symptoms and diseases were obtained from clinical records. In addition, a detailed history of smoking habit, respiratory symptoms, and diseases was recorded directly from the patients with the aid of their parents. A complete clinical physical examination and lung function tests were then performed in all patients.
CLINICAL DATA COLLECTION
Twenty five patients had suffered from acute lymphoblastic leukaemia (ALL), 22 acute myelogeneous leukaemia (AML), and five chronic myelogeneous leukaemia (CML). Patients with AML were treated according to different cooperative protocols of the Italian Association of Paediatric Haematology/Oncology (AIEOP).8 ,9 In particular, all children received an identical induction therapy including daunorubicin (DNR) and cytosine arabinoside (Ara-C) according to the classical scheme 3+7, followed by a second course of two days DNR and five days Ara-C. One or two courses of consolidation therapy with high dose Ara-C, etoposide, and mitoxantrone were administered before autologous or allogeneic BMT.
Children with ALL received different AIEOP first line protocols—namely, 8503 or 8703, which were based on the experience of studies like those of the Children Cancer Group, and 8803 and 9103 which were based on the Berlin-Frankfurt-Munster stratification and treatment criteria.10-12 These protocols included multiple cytotoxic drugs such as prednisone, vincristine, DNR, low and high dose methotrexate, Ara-C, l-asparagimase, and 6-mercaptopurine. Details of the above two year front line treatments are reported elsewhere.10-12
Bone marrow transplantation was performed in 23 patients during their first complete haematological remission and in 24 patients during their second or third remissions following medullary relapses. Patients with CML were considered to be comparable to patients given BMT during the first remission on the basis of the usually mild previous chemotherapy received.
Nineteen patients were given autologous bone marrow transplants and 33 received allogeneic transplants.
Thirty nine patients had received a conditioning regimen including fractioned total body irradiation (12 Gy divided into six fractions over three days), 11 had received a conditioning regimen including busulphan, and two a regimen of BCNU, amsacrine, Ara-C, and VP16.
After BMT 25 children developed acute GVHD (grade I–III) and eight developed chronic GVHD. Acute GVHD was classified according to previously described criteria13 and was treated with steroids as first line therapy and horse antilymphocyte globulin in resistant cases. Chronic GVHD was diagnosed according to the criteria published by Shulman14 and treated with cyclosporin A and steroids when necessary.
Thirty of the 33 patients given an allogeneic transplant had a positive serological test for human cytomegalovirus (HCMV) prior to BMT. Seven patients had HCMV reactivation. Eight patients developed at least one episode of pulmonary infection: one had two episodes (one about three years after BMT), one had one episode of pneumonia and subsequently two episodes of acute bronchitis, one had four episodes of pneumonia (the last two years after BMT), and one developed pulmonary aspergillosis. Acute bronchitis was defined as an acute episode of airway inflammation with cough and mucopurulent phlegm requiring treatment; pneumonia was defined as the occurrence of an acute pulmonary infection with physical and radiographic signs of parenchymal shadowing. Isolation of bacterial, mycotic and viral agents from secretions obtained by deep coughing was attempted.
The clinical features of the patients included in this study are summarised in table 2.
Clinical features of patients included in the study
RESPIRATORY SYMPTOMS
The presence of cough and/or phlegm, dyspnoea, and wheezing both at rest and during exercise was recorded at follow up appointments, the details coming directly from the patients or from their parents. Information on the entire period following transplantation was obtained from clinical records. Patients had been routinely visited every three months after BMT.
PULMONARY FUNCTION TESTS
Measurements of lung volumes were obtained using a water sealed spirometer (Pulmonet III, Sensor Medics, Anaheim, California, USA) and were performed according to the European Community for Coal and Steel (ECCS) statements15 and the American Thoracic Society (ATS) recommendations.16 The best of three forced vital capacity (FVC) measurements was recorded as well as forced expiratory volume in one second (FEV1), FEV1/FVC ratio, and maximal expiratory flow at 25% of FVC (MEF25).
Transfer factor for carbon monoxide (Tlco) was determined using the single breath method (Transferscreen-II Jaeger, Wuerzburg, Germany) and corrected for haemoglobin content. Since the correction of Tlco for alveolar volume did not influence the results of our analysis, only uncorrected Tlco values are reported. Measurements were performed according to the ECCS15 and ATS17 guidelines. Since this test is more difficult and requires greater cooperation to perform than FVC, Tlco data are unavailable for six patients.
Functional data were expressed as an SD score (actual result—predicted values/population standard deviation) and defined as pathological when less than –1.64 corresponding to less than the 5th percentile. Taking into account the pubertal stage of each subject evaluated according to Tanner’s method, the SD score was corrected according to the tables reported by Rosenthal et al.18Reference values used were those from a recent cross sectional study on lung function in healthy schoolchildren aged 4–19 years.19 ,20
Three patterns of respiratory function abnormalities were defined as follows: (1) restrictive (FVC SD score less than –1.64 with FEV1/FVC SD score ratio more than –1.64); (2) obstructive (FEV1 SD score less than –1.64 with FEV1/FVC SD score ratio equal to or less than –1.64); (3) isolated diffusing impairment: (Tlco SD score less than –1.64 and other parameters in the normal range).
STATISTICAL ANALYSIS
χ2 tests for independent samples were applied to verify the null hypothesis of independence between the lung function abnormalities and a series of clinical and treatment parameters. As dependent variables we considered the pathological respiratory function, grouping all the three above mentioned patterns versus the normal one. As independent variables we considered disease status at the time of BMT, subdividing children into those given transplantation during the first remission and those transplanted in more advanced disease, age at BMT, period of time in which BMT was performed (before or after 1990), time interval between BMT and cross sectional observation, type of BMT, inclusion of total body irradiation (TBI) in the conditioning regimen, presence of acute and/or chronic GVHD, reactivation of HCMV infection, and the presence of pulmonary infections.
Results
At examination patients were found to be free of acute rejection, infection, active GVHD, or any other acute disease. None of the patients smoked and no patient reported chronic respiratory symptoms. The young subjects who were unable to collaborate with the lung function testing did not have any chronic respiratory symptoms according to their parents.
Table 3 reports the mean (SD) values of the functional parameters and percentages of patients with SD scores still in the normal range but negative (between 0 and –1.64) and of those with clearly pathological SD scores (less than –1.64). The mean values of all the functional parameters were still in the normal range although FVC, FEV1 and, in particular, Tlco were negative; in fact, there was a significant proportion of patients with negative and clearly pathological FVC and Tlco values.
Mean (SD) values of lung function parameters and percentages of patients with negative SD scores and clearly pathological SD scores
The distribution of respiratory function patterns showed that only 62% of patients had normal respiratory function. No patient had an obstructive pattern, 23% had a restrictive pattern of whom 44% also had a pathological Tlco, and a substantial group of patients (15%) had an isolated impairment of Tlco with normal lung volumes. When patients were grouped according to the type of BMT, the percentage with a normal pattern greatly increased in the group that had received an autologous BMT (74%). In fact, no patient in this group had an isolated impairment of Tlco (fig1).
Distribution of respiratory function patterns in (A) all the study patients and (B) in those receiving allogeneic and autologous bone marrow transplantation (BMT).
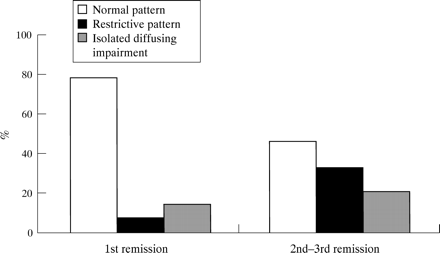
As far as the associations between the different variables and lung function abnormalities are concerned, the status of the underlying disease at the time of BMT was found to have a statistically significant effect (p<0.02). The percentage of patients with lung function abnormalities was significantly higher in the patients who received a bone marrow transplant after two or three complete remissions compared with those who were transplanted immediately after achieving the first remission (54% vs 21%; fig 2). All patients with CML were analysed together with children with acute leukaemia given a bone marrow transplant in the first remission and all had normal lung function.
Distribution of respiratory function patterns in patients receiving bone marrow transplantation (BMT) during first remission and in those transplanted after the second or third remission.
The percentage of patients with lung function impairment was higher (and nearly statistically significant) in those who underwent allogeneic BMT than in those who underwent an autologous transplantation (45% vs 26%; p = 0.06), and in those with pulmonary infections than in those without (56% vs 26%; p = 0.07). All but one patient with pulmonary infections received an allogeneic transplant. Allogeneic BMT resulted in an increase in lung function abnormalities compared with autologous BMT, even in the absence of respiratory infections (42% vs 21%). All four subjects who had received a bone marrow transplant in the second or third complete remission and who had suffered from a pulmonary infection (three from pneumonia and one from aspergillosis) had impaired lung function.
The occurrence of long term abnormalities of lung function was not significantly associated with age at BMT, year of BMT, or time interval between BMT and cross sectional observation. It was not associated with the type of conditioning regimen, the percentage of subjects with lung function abnormalities being not significantly different in subjects receiving TBI (41%) and those receiving busulphan (27%), nor was it associated with occurrence of acute or chronic GVHD or HCMV reactivation after BMT.
Discussion
Our results show that more than one third of survivors of childhood BMT have subclinical abnormalities of lung function. In fact, in spite of the absence of chronic respiratory symptoms, only about 60% of our patients had respiratory function within normal limits a mean of five years after BMT. The percentage of patients with lung function abnormalities in our sample was similar to that of Nysomet al 6 but slightly lower than that reported in a study by Nenadov et al.5 This difference could be explained by the fact that patients included in this study were affected by solid tumours and had been heavily retreated with conventional chemotherapy, surgery, and radiotherapy before receiving the conditioning procedure. Moreover, the sample of surviving patients was biased by the very poor prognosis of these diseases (53 patients alive vs 350 transplanted). An interesting common result was, however, the scarcity of respiratory symptoms even in advanced disease.
Our large sample size allowed us to examine the effect of several potential risk factors, including type of transplant, on lung function abnormalities. We were able to establish that to receive BMT during the second or third remission would require a larger amount of chemotherapy as first line treatment, and this appears to be the most significant determinant of more permanent lung injury. In contrast, receiving less chemotherapy, as in patients with CML or those receiving BMT after a first remission, appears to be a significant factor in preserving lung function. These results support our previous conclusions7on the correlation of toxicity with first line therapy. Unfortunately the number of children receiving BMT for non-malignant disorders in our cohort of patients was too small to allow us comparisons that would have provided a more definitive statement. In addition, there appear to be different individual susceptibilities to drug toxicity that precludes a precise identification of the subjects at greater risk.
Allogeneic BMT with consequent immunosuppression and more frequent pulmonary infections can contribute to impairment of lung function. A possible explanation for the higher incidence of lung function abnormalities observed in recipients of allogeneic bone marrow transplants compared with children given autologous transplants could be immune mediated lung damage caused by donor lymphocytes.
Both busulphan and TBI in the conditioning regimen are known to cause lung toxicity; we did not find any significant difference between them, unlike Beinert et al 21 who found a significantly higher incidence of long term lung impairment with busulphan conditioning than with TBI; it is possible that our data are biased by the non-homogeneous distribution of the type of conditioning in our sample.
Unfortunately our study is cross sectional and lacks baseline respiratory function data. However, given the young age at which the patients developed their disease, it is unlikely that respiratory function was already abnormal. There is one longitudinal study with a long term follow up6 but this, too, lacks pulmonary function tests before transplantation for most of the patients. The homogeneous population sample (all patients had received allogeneic transplants) and the relatively small number of patients did not allow all risk factors to be evaluated.
We conclude that, in many patients, lung function abnormalities are present many years after childhood BMT for haematological malignancies even in the absence of chronic respiratory symptoms. After BMT regular monitoring of lung function in all subjects is recommended and, in particular, in those at higher risk of lung injuries such as those with more than one remission, those receiving allogeneic BMT, and those having suffered from pulmonary infections. If lung function abnormalities become apparent, long term follow up is necessary in order to see whether they become clinically relevant. As recipients of bone marrow transplants have a long life expectancy and may have to cope with risk factors such as smoke, pollution, infections and ageing in addition to their possible limited pulmonary function reserve, they should remain non-smokers and have early and aggressive treatment for respiratory illness, perhaps including pneumococcal vaccination.
Acknowledgments
Grant support: Current Research Project, IRCCS, Policlinico S. Matteo, Pavia, No. 681RCR96/02.