Article Text
Abstract
COPA (coatomer subunit α) syndrome is a newly recognised cause of interstitial lung disease in children and adults, frequently associated with arthritis and renal dysfunction. We report a 11-year-old girl with disease limited to major pulmonary haemosiderosis manifesting at the age of 2 years, due to a heterozygous p.(Arg233His) mutation in COPA. Her interferon (IFN) signature was elevated (10.312 and 12.429, healthy <2.466), as was the level of serum IFNα (211 fg/mL, healthy <10 fg/mL). STAT1 phosphorylation in T lymphocytes and monocytes was increased as compared with healthy controls. Based on these results she was treated with the JAK1/2 inhibitor ruxolitinib, which resulted in reduction in IFN signalling and appeared to be associated with partial though incomplete decrease in the severity of her pulmonary disease. Patients with alveolar haemorrhage of unknown origin should be considered for COPA screening. Functional tests can help to personalise patient therapy.
- paediatric interstitial lung disease
- rare lung diseases
- systemic disease and lungs
- massive haemoptysis
- paediatric lung disaese
Statistics from Altmetric.com
- paediatric interstitial lung disease
- rare lung diseases
- systemic disease and lungs
- massive haemoptysis
- paediatric lung disaese
Introduction
Coatomer subunit α (COPA) syndrome is a recently described, rare, multisystemic, likely immune-mediated disorder. It is caused by heterozygous mutations in COPA, encoding the subunit alpha of the coatomer complex-I that ensures the retrograde trafficking of proteins from the Golgi to the endoplasmic reticulum (ER). Families segregating pathogenic mutations in COPA reported to date are characterised by variable phenotypic expression and a significant degree of non-penetrance (online supplementary table 1). Clinically, COPA syndrome manifests as a disease of lungs, joints and kidneys, mainly in childhood, but also sometimes in adulthood.1 Chronic alveolar haemorrhage is the most common pulmonary manifestation, but interstitial lung disease (ILD), evolving towards lung fibrosis with traction cysts and follicular bronchiolitis, has also been described.2 A majority of reported patients have demonstrated joint involvement; this can be severe, with a polyarticular destructive disease affecting mainly the knees, shoulders and hands, and has been variably categorised as juvenile idiopathic arthritis or rheumatoid arthritis with positive rheumatoid factor (RF). Other features include positive antineutrophil cytoplasmic antibodies (ANCA) and antinuclear antibody titres, proliferating and fibrosing glomerulonephritis, autoimmune thyroiditis and neurological symptoms.1 Previously, patients have been treated with corticosteroids and immunosuppressive drugs, often with a positive initial effect. However, an evolution towards terminal respiratory insufficiency and lung transplantation is a major characteristic.
Supplemental material
To date, all reported cases of COPA syndrome harbour one of five missense mutations in a hot spot spanning exons 8–9 of the COPA gene, encoding the WD40 domain of the protein.1 As observed in the present report, an increased expression of type 1 interferon (IFN)-stimulated genes (ISGs) has also been described in affected individuals, suggesting that blocking IFN signalling might provide therapeutic benefit.3
Clinical case
Patient presentation
The patient was the first child of an unrelated couple. From the age of 2 years she experienced repeated episodes of severe anaemia (haemoglobin (Hb) nadir of 20 g/L), and experienced five distinct acute respiratory events in early childhood, characterised by cough and the presence of alveolar opacities on chest X-ray that were attributed to viral infection. At age 4 years, as her anaemia was thought to be related to a haematological disorder, she received a short oral course of corticosteroid with transient reported efficacy. However, 3 months thereafter she experienced a first episode of haemoptysis, leading to a diagnosis of alveolar haemorrhage. High-resolution (HR) CT scan showed bilateral patchy ground glass opacities, and bronchoalveolar lavage revealed Hb laden macrophages with a Golde score of 166 (figure 1A–C). Given that idiopathic pulmonary haemosiderosis has been associated with autoimmune disease, an extensive immunological exploration was performed. A slight CD8 and NK lymphopenia, a mildly positive RF with no clinical signs of articular involvement, and a threshold titre of 1/100 antismooth muscle antibody were documented. Other autoantibodies were negative, including ANCA. Neither coeliac disease nor cow’s milk allergy was found. A lung biopsy (figure 1D) was performed by thoracotomy and demonstrated heterogeneous stigmata of intra-alveolar bleeding with hemophagocytosis. However, the parenchymal structure was preserved with no distortion, thin alveolar walls, no fibrosis, and no sign of vasculitis or pulmonary hypertension. Very few lymphoid nodules were present (online supplementary figure 1).
Supplemental material
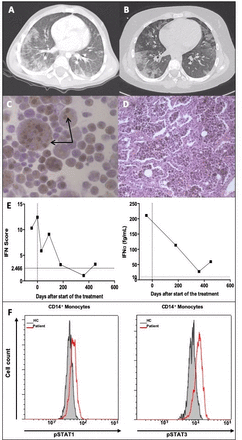
(A, B) Thoracic high-resolution (HR) CT-scan evolution. (A) First thoracic HR-CT scan at age 4 years demonstrating diffuse patchy ground glass opacities in the absence of lung fibrosis or vascular abnormalities. (B) At age 10 years there was a persistence of diffuse ground glass and alveolar opacities. (C) Initial bronchoalveolar lavage (BAL). Most of the cells retrieved at BAL were haemosiderin laden macrophages (siderophages), suggestive of a chronic alveolar haemorrhage. (D) Surgical lung biopsy. H&E staining. At high magnification the parenchymal structure was preserved, with thin alveolar walls, and no features of pulmonary arterial hypertension, lung fibrosis, vasculitis or other inflammatory infiltrate. Haemosiderin-laden macrophages (siderophages) filled the alveolar spaces. The biopsy showed very rare lymphoid nodules (online supplementary figure 1). (E, F) Constitutive activation of type I IFN signalling observed in the patient before and during treatment. (E) IFN score calculated from the median fold change in relative quantification values of a set of six IFN stimulated genes (IFI27, IFI44L, IFIT1, ISG15, RSAD2, SIGLEC1, normal <2.466) in the peripheral blood from the patient, before and during treatment (left panel). Concentrations of IFNα protein were assessed by ultra-sensitive digital ELISA (healthy controls <10 fg/mL) in plasma or serum from the patient before and after starting treatment with ruxolitinib (right panel).5 The vertical dotted line indicates the initiation of treatment. (F) Increased constitutive phosphorylation of STAT1 (left panel) and STAT3 (right panel) in monocytes from the patient compared with a healthy control. Similar results were observed in CD3+, CD4+ and CD8+ lymphocytes (online supplementary figure 3).
Supplemental material
Subsequent disease evolution was marked by multiple episodes of pulmonary decompensation secondary to alveolar haemorrhage. Disease was initially controlled with corticosteroids (oral and intravenous pulses). However, at age 7 years the patient experienced a severe episode requiring extra-corporeal membrane oxygenation for 15 days, followed by an increased frequency of relapses up to once a month. Immunosuppressive drugs (hydroxychloroquine, azathioprine, mofetil mycophenolate and cyclophosphamide) were trialled with limited effect.4 In between episodes, exercise tolerance was severely limited and schooling was significantly compromised.
Genetic and functional analyses
At 11 years of age targeted next generation sequencing identified a deleterious heterozygous COPA mutation in exon 8, c.698G>A; p.(Arg233His),1 which was inherited from the child’s asymptomatic mother (online supplementary figure 2). Considering the severity of the phenotype, and the refractoriness to multiple lines of treatment, we investigated the type I IFN pathway with a view to targeted therapy. An IFN signature was elevated on two occasions (10.312 and 12.429, healthy <2.466). Ultra-sensitive digital ELISA using highly specific pan-IFNα antibodies, revealed an elevated level of serum IFNα protein (211 fg/mL, healthy <10 fg/mL) (figure 1E and online supplementary figure 3),5 and STAT1 and STAT3 phosphorylation was increased in T lymphocytes and monocytes as compared with a healthy control (figure 1F and online supplementary figure 3).
Supplemental material
Disease evolution
Based on the above results we trialled the use of a JAK1/2 specific inhibitor, Ruxolitinib (15 mg two times per day, ie, 0.5 mg/kg/day). After 3 months of therapy it became possible to wean the patient off corticosteroids, and she reported a dramatic improvement in her well-being and quality of life, mirrored by a complete absence of hospitalisations and school absenteeism, and an ability to cycle for up to 1 hour without experiencing dyspnoea. In total, only two, mild, exacerbations occurred over a 1-year period of ruxolitinib treatment, with a Hb nadir of 100 g/L, versus a baseline Hb of 120 g/L. On both occasions the child was treated with a 5-day oral course of corticosteroids. Despite such a marked clinical improvement, evaluation 12 months after the initiation of ruxolitinib indicated a degree of continued chronic alveolar bleeding. Reticulocyte count ranged between 80 000 and 120 000/mm3 despite a stable Hb, and HR-CT scanning revealed a persistence of alveolar opacities resulting from dense ground glass opacities and the presence of infra-centimetric subpleural traction cysts suggesting a slow progression towards lung fibrosis. Pulmonary function tests were stable, with a moderate restrictive pattern and altered gas diffusion. Ruxolitinib was well tolerated. The IFN score and the IFNα protein level showed a trend to reduction (figure 1E).
Discussion
Since the first description in 2015, only 30 patients with COPA syndrome have been reported, including 11 patients harbouring the p.(Arg233His) mutation identified in the proband discussed here. In all previous cases there was multisystem disease affecting at least the lung and the joints. It is of note then that in the described patient, phenotypic expression was apparently limited to the lungs, manifesting as early onset and severe disease, with alveolar haemorrhage being the main feature. No lung comorbidity nor environmental exposure was identified to explain the severity of her lung disease. The mother of the proband also carries the same pathogenic mutation in COPA. Despite this, she demonstrates no clinical signs or lung function abnormality, an observation consistent with some previously reported familial cases. Of note, the mother exhibited a mildly positive type I IFN signature and slightly increased serum IFNα protein on two occasions (online supplementary figure 3). The maternal grandmother was found not to carry the mutation. The maternal grandfather, a non-smoker, died from lung cancer.
Idiopathic pulmonary haemosiderosis in children is a very rare disorder which has been associated with a high frequency of autoimmune disease in a large French cohort ascertained through a nationwide reference network for rare lung diseases (RespiRare).4 Of 34 patients with haemosiderosis identified through the RespiRare collaboration, 10 patients with available DNA were tested for COPA mutations, with the identification of the single patient presented here. Autoinflammatory disorders are a relatively recently described cause of childhood ILD (chILD) and alveolar haemorrhage.6 Thus, children with alveolar haemorrhage or chILD of unknown origin should be considered for COPA sequencing. The diagnostic yield is likely to be higher where other features coexist.
COPA syndrome was originally hypothesised to result from abnormal protein trafficking leading to ER stress, induction of an unfolded protein response and priming of T helper (Th) 17 signalling. However, it remains to be determined how these mechanisms result in alveolar haemorrhage, particularly in cases such as this where there was no histological evidence of vasculitis and a relative absence of other histological findings. More recently, mutations in COPA have been associated with an increased expression of ISGs in blood circulating cells of affected patients.3 Consistently, we observed enhanced type I IFN signalling in the patient. Thus, the pathophysiological mechanisms of the mutant COPA-associated phenotype are not understood, in particular the relative contribution of type I IFN activation to pathogenesis. Although COPA is a ubiquitously expressed protein, the explanation for the observed pattern of organ involvement is unclear. Incomplete penetrance might suggest that a ‘second-hit’ is required to initiate disease onset and/or drive disease progression. Despite an absence of definitive evidence, viral infections are often considered as precipitating factors in chILD. We observed a lower IFN score and lower levels of IFNα protein in the asymptomatic mother of the proband, perhaps suggesting that a trigger is necessary for clinical disease to manifest.
To our knowledge, this is the first report of the use of ruxolitinib in a child with life-threatening haemorrhagic lung disease in the context of COPA syndrome. Assessment of the type I IFN pathway allowed us to personalise therapy using JAK1/2 inhibition, with an aim to target putative IFN-driven autoinflammation. Even if positive changes in reticulocyte count, lung function and the appearances on HR-CT scanning were not obvious, the important clinical improvement, and trend towards reduction of both the IFN score and the level of IFNα protein during treatment, suggest that JAK1/2 inhibition should be considered as a therapeutic option to control lung disease in COPA syndrome, perhaps in combination with other anti-inflammatory molecules. Our findings also raise the possibility that the extra-pulmonary aspects of the COPA syndrome phenotype might similarly benefit from such a therapeutic approach.
Acknowledgments
We thank the patient and her family for their enthusiastic participation in this study. We thank the Assistance Publique Hôpitaux de Paris, Sorbonne Université, Paris, France, and the national networks for rare lung diseases: Centre de référence des maladies respiratoires rares (RespiRare), Centre de référence des maladies pulmonaires rares (OphaLung) and Filière de soins pour les maladies respiratoires rares (RespiFIL). The ILD cohort has been developed in collaboration with the Rare Disease Cohort (RaDiCo)-ILD project (ANR-10-COHO-0003), the FP7-305653-child-EU project and the COST Action European network for translational research in children's and adult interstitial lung disease (COST-ILD) project (CA16125). YJC acknowledges the European Research Council (GA 309449), and a state subsidy managed by the National Research Agency (France) under the ’Investments for the Future’ programme bearing the reference ANR-10-IAHU-01. YJC and DD acknowledge the ANR (grant CE17001002). We thank Immunoqure AG for provision of mAntibodies for the Simoa assay.
Footnotes
Contributors M-LF performed the functional studies under the direction of YC; NN was in charge of the patient and the treatment monitoring in the Reference Center headed by AC. ML and NN were responsible for the genetic studies under the direction of SA. SR, MF, LB, NR, BN participated in the care of the patient. CS, HDLP, AC, HB reviewed the imaging and histological data. GIR, DD and VB participated in the functional studies. M-LF and NN wrote the manuscript, which was reviewed by all of the authors.
Funding Our work is supported by grants from the Institut National de la Santé et la Recherche Médicale (Inserm, reference: 000427993 for M-LF), the Legs Poix from the Chancellerie des Universités (grants 2013 n°1305, 2014 n°1405, 2015 n°1015, 2016 n°2077 and 2017 n°DP2017/1860), Paris, the European Union’s Seventh Framework Program (FP7-ChILD-EU 2007-2013) under grant agreement n°305653, as well as funding from the patient organisations 'Respirer c’est Grandir' and 'Belleherbe Association'.
Competing interests None declared.
Patient consent for publication Parental/guardian consent obtained.
Provenance and peer review Not commissioned; externally peer reviewed.